Figures & data
Figure 1 Mycobacterium avium paratuberculosis within macrophage. MAP infects by passing the mucosal barrier, preferentially via M cells, and after which it is engulfed by subepithelial macrophage (A). A number of MAP bacilli are degraded within the phagosome (B) and stimulate CD4+ immune responses via antigen presentation through the MHC class II pathway. (C) Alternatively MAP evades destruction within phagosome, through inhibition of normal killing mechanisms, and proliferates. (D) Secreted MAP proteins may pass from the phagosome to the cytosol and be subsequently available for presentation to CD8+ immune responses via the MHC class I pathway. Survival and proliferation within macrophage is a vital element in the virulence of paratuberculosis.
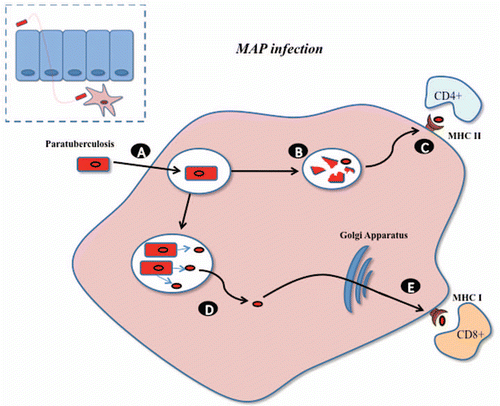
Figure 2 Infectious life cycle of L. monocytogenes (adapted from Sleator and Hill). Listeria has the ability to cross the epithelial barrier, through the expression of internalins (inlA, inlB), and after which be engulfed by sub-epithelial macrophage (A). While in the phagosome a number of cells are lysed (B) releasing antigens for subsequent presentation through the MHC class II pathway to stimulate CD4+ immune responses (C). Alternatively, Listeria escapes the phagosome using a pore forming cytolysin called listeriolysin O (D). Within the cytosol, Listerial proteins may be processed via the MHC class I pathway for stimulation of CD8+ immune responses (E). Listeria may also pass to neighboring cells through the expression of other virulence factors (F).
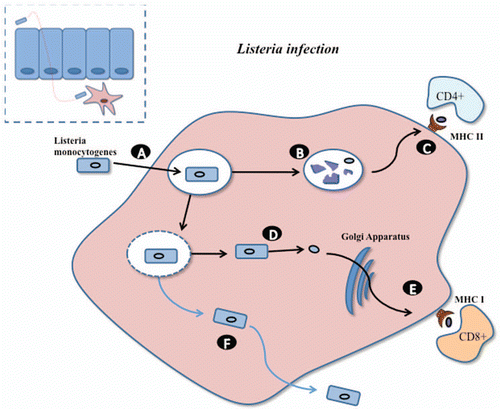
Figure 3 Novel patho-biotechnological vaccine development against Johne disease. A Lactobacillus strain, harbouring an expression vector encoding MAP antigens, will be equipped with an internalin from Listeria which will allow translocation across the epithelial barrier and subsequent phagocytosis by macrophages (A). In a similar manner to Listeria within the phagosome, a number of lactobacilli will be lysed allowing presentation of the expressed MAP antigens via the MHC class II pathway to stimulate strong CD4+ immune responses (B and C). Heterologously expressed listeriolysin O, from Listeria monocytogenes, will allow the Lactobacillus strain to escape the phagosome. (D) Expression of MAP antigens within the macrophage cytosol will stimulate strong CD8+ immune responses through the MHC class I pathway (E).