Figures & data
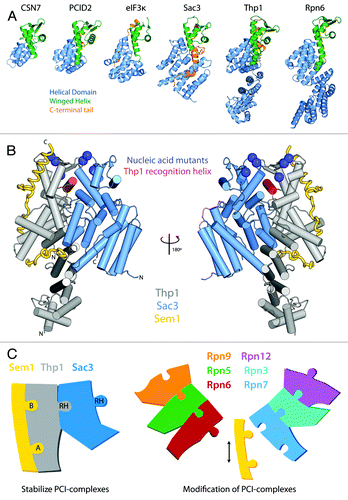
Figure 1. (A) Structure of PCI proteins currently available. PCI-proteins contain a bipartite fold consisting of a N-terminal helical domain (blue) and a C-terminal winged-helix domain (green). The C-terminal winged-helix domain often leads to a C-terminal tail (orange) that interacts with the surface of the helical domain. (B) The Sac3-Thp1-Sem1 structure. The two PCI-proteins Thp1 (gray) and Sac3 (blue) share an extensive interaction interface marked by the insertion of the Thp1 WH “recognition helix” (red) into a cleft formed by the Sac3 WH and HD. The small Sem1 protein (yellow) wraps around the surface of Thp1 and makes little contact with Sac3. Blue spheres indicate the position of side-chains of conserved positive residues that when mutated to a negative charge, resulted in a loss of nucleic acid binding and mRNA export defects in yeast. (C) The role of Sem1 in modulating PCI complexes. Sem1 acts to stabilize the mini-PCI complex formed between Sac3-Thp1-Sem1 by binding to two sites on the Thp1 surface (Sites A and B). In doing so it may be acting as a capping protein shielding the surface of Thp1 from interacting with other PCI-proteins. Within the larger PCI-complex of the 26S proteasome lid Sem1 may act as a modulator of complex assembly, or subunit composition, by binding to and capping specific PCI-protein subunits. Within the schematic representations, the recognition helix (RH) of each WH domains is shown as a protruding tab.