Figures & data
Figure 1. The pH and time dependency of human neutrophil elastase activity. Shown are the NE activity optima according to the pH value of the buffer solution (A) as well as kinetic studies in the in vitro model system using pure substances (B) and in the supernatant of PMNs (C). An amount of 68 nM NE and 1 mM substrate were incubated with different pH values and in a time-dependent way. The pH optimum of NE activity could be approved in the neutral range (between pH 7–8). The supernatant of 3 × 105 PMNs (PMA-activated) was incubated with 1 mM substrate and NE activity was determined in a time-dependent way. In traces (B and C) results obtained at pH 5 are indicated as squares, wheras results obtained at pH 7.5 are indicated as circles. The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation.
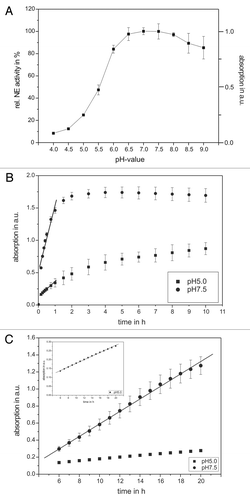
Figure 2. The pH-dependency of human antitrypsin. The pH-dependent influence of AT on the NE activity in an in vitro model system (A) and in the supernatant of activated PMNs (B) are presented. Sixty-eight nanomolars NE and 1 mM substrate were incubated with AT in a concentration dependent way (0.095–1.9 µM) for 20 min at pH 7.5 (inverse triangles) and pH 7 (triangles) as well as for 60 min at pH 6 (circles) and pH 5 (squares), respectively, at RT (trace A). PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate and AT in a concentration dependent way (0.095–1.9 µM) (circles) and pH 5 (squares) at RT (trace B). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
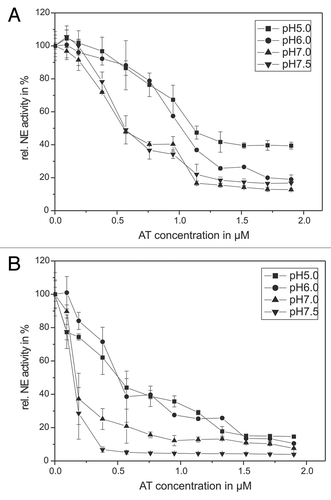
Figure 3. Shown is the impairing influence of HOCl (A) and the MPO-H2O2-Cl−-system (B) on the AT inhibiting activity toward NE. Sixty-eight nanomolars NE, 1 mM substrate and 1.14 µM AT were incubated with HOCl in a concentration dependent way (1–15 µM) for 20 min at pH 7.5 (gray bars) and for 60 min at pH 5 (black bars), respectively, at RT (trace A). An amount of 68 nM NE, 1 mM substrate and 1.14 µM AT were incubated with the MPO-H2O2-Cl−-system in a concentration dependent way (1–10 nM MPO, 2–20 µM H2O2, 140 mM NaCl) for 60 min at pH 7.5 (gray bars) and pH 5 (black bars), respectively, at RT (trace B). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of 3 independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
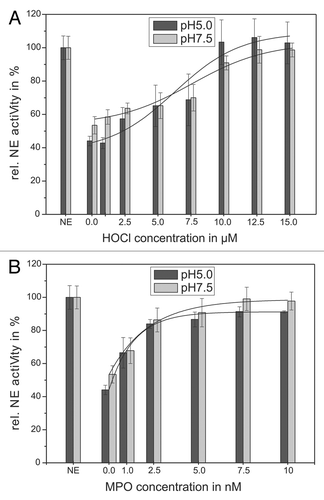
Figure 4. Shown is the protection of the AT inhibitory activity toward NE by means of l-methionine. 68 nM NE, 1 mM substrate, 12.5 µM HOCl and 1.14 µM AT (pH 5, black bars) or 0.57 µM AT (pH 7.5, gray bars), respectively, were incubated with l-methionine in a concentration dependent way (0.1–1 mM) for 20 min at pH 7.5 (gray bars) and 60 min at pH 5 (black bars) at RT (trace A). The HOCl addition is marked with stripes. An additional NE activity reducing effect of l-methionine could be observed without any HOCl addition (trace B). PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate, 1.14 µM AT (pH 5, black bars/squares) or 0.57 µM AT (pH 7.5, gray bars/circles), respectively, and l-methionine in a concentration dependent way (0.05–500 µM) for 16 h at pH 7.5 (circles) and pH 5 (squares) at RT (trace C). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
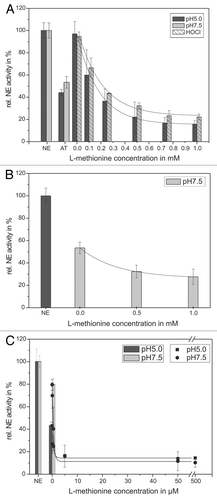
Figure 5. Shown is the protection of the AT inhibitory activity toward NE by means of ASA. 68 nM NE, 1 mM substrate, 12.5 µM HOCl and 1.14 µM AT (pH 5, black bars) or 0.57 µM AT (pH 7.5, gray bars), respectively, were incubated with ASA in a concentration dependent way (0.1–1 mM) for 20 min at pH 7.5 (gray bars) and 60 min at pH 5 (black bars) at RT (trace A). The HOCl addition is marked by stripes. PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate, 1.14 µM AT (pH 5, black bars/squares) or 0.57 µM AT (pH 7.5, gray bars/circles), respectively, and ASA in a concentration dependent way (0.01–1 mM) for 16 h at pH 7.5 (gray bars/circles) and pH 5 (black bars/squares) at RT (trace B). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
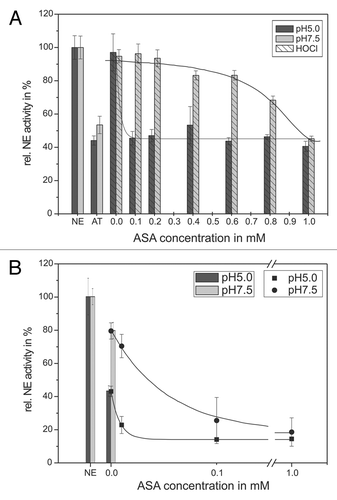
Figure 6. Shown is the protection of the AT inhibitory activity toward NE by means of cefoperazone. 68 nM NE, 1 mM substrate, 12.5 µM HOCl and 1.14 µM AT (pH 5, black bars) or 0.57 µM AT (pH 7.5, gray bars), respectively, were incubated with cefoperazone in a concentration dependent way (0.05–0.6 mM) for 20 min at pH 7.5 (gray bars) and 60 min at pH 5 (black bars) at RT (trace A). An additional NE activity reducing effect of cefoperazone can be observed, without AT addition at pH 7.5 (trace B). PMNs (5 × 106) were activated with PMA (10−7 M) for 30 min at 37°C and the supernatant was incubated with 1 mM NE substrate, 1.14 µM AT (pH 5, black bars/squares) or 0.57 µM AT (pH 7.5, gray bars/circles), respectively, and cefoperazone in a concentration dependent way (2–200 µM) for 1 6 h at pH 7.5 (gray bars/circles) and pH 5 (black bars/squares) at RT (trace C). The NE activity was spectrophotometrically determined at 410 nm. Shown are the mean values of three independent experiments ± standard deviation. The NE activity without AT addition was set to 100% and used as reference value (%).
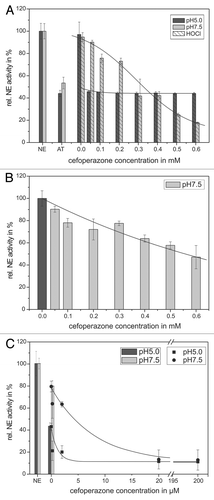
Figure 7. Shown is the influence of AT on PMN vitality. 106 PMNs were co-incubated with AT (0–1.9 µM) in RPMI1640 medium (10% FBS) for 16 h at 37°C. Apoptosis and vitality were investigated by means of annexin V-FITC and PI binding. PMNs were quantitatively analyzed by flow cytometry. PMNs without treatment show increased annexin V-FITC binding after 16 h inubation [trace (A)], but co-incubation with AT could not cause any additionally effect [trace (B and C)]. The number of PI-positive is cells is marginal for untreated cells as well as for AT-treated PMNs [traces (A and C)]. Shown are the mean values of three independent experiments ± standard deviation.
![Figure 7. Shown is the influence of AT on PMN vitality. 106 PMNs were co-incubated with AT (0–1.9 µM) in RPMI1640 medium (10% FBS) for 16 h at 37°C. Apoptosis and vitality were investigated by means of annexin V-FITC and PI binding. PMNs were quantitatively analyzed by flow cytometry. PMNs without treatment show increased annexin V-FITC binding after 16 h inubation [trace (A)], but co-incubation with AT could not cause any additionally effect [trace (B and C)]. The number of PI-positive is cells is marginal for untreated cells as well as for AT-treated PMNs [traces (A and C)]. Shown are the mean values of three independent experiments ± standard deviation.](/cms/asset/61e12d39-258c-4495-97e4-df5699c5347e/kbim_a_10919190_f0007.gif)
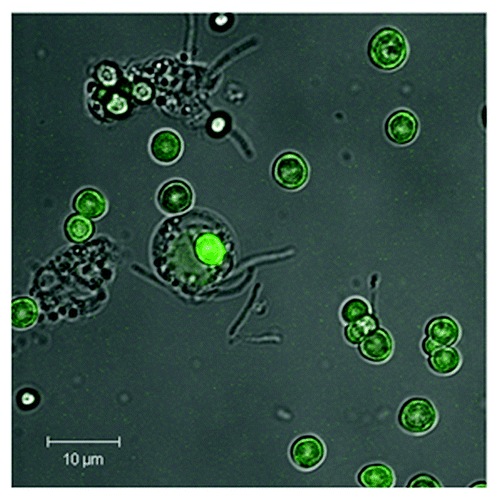
Figure 8. Shown is the phagosomal uptake of AT-functionalized CaCO3 microcarriers by PMNs. CaCO3 microparticles were coated with five layers PRM/DXS and surface-coated with FITC-labeled AT according to the following scheme CaCO3//PRM/DXS/PRM/DXS/PRM/AT-FITC. A uniform AT-FITC coating can be seen by means of CLSM. Microparticles (5 × 105) were co-incubated with PMNs (105) in a ratio of 5:1 for 24 h (37°C, 5% CO2). Uptake by PMNs was visualized by means of CLSM. A representative result of more than five independent measurements is presented.