Figures & data
Figure 1. Strategies for in vivo secretion of therapeutic antibodies: direct injection of genetic material using non-viral or viral vectors (in vivo gene therapy), and implantation of genetically modified cells (ex vivo gene therapy).
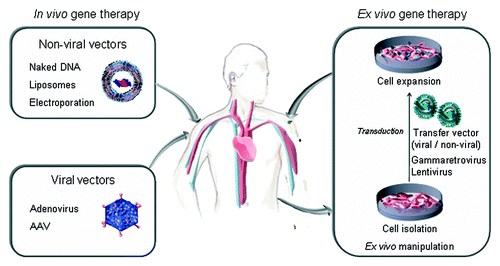
Table 1. Ex vivo gene-modified mesenchymal stem cells for cancer immunotherapy
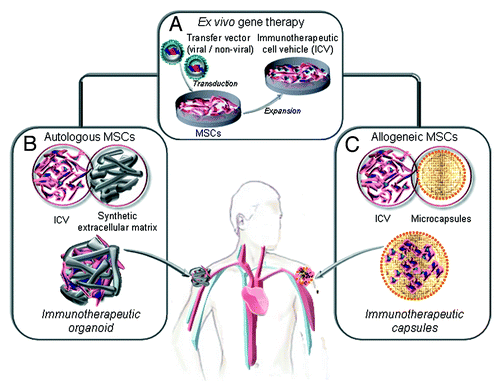
Figure 2. Ex vivo generation of genetically modified mesenchymal stem cells (MSC) as factories for long-term in vivo secretion of immunotherapeutic proteins. (A) Ex vivo gene therapy of autologous or allogeneic MSC (isolation, expansion and lentiviral transduction) to generate an immunotherapeutic cell vehicle (ICV). (B) The autologous ICV is embedded in a non-immunogenic synthetic extracellular matrix and implanted by subcutaneous injection (immunotherapeutic organoids). The allogenic ICV is microencapsulated and implanted by subcutaneous injection (immunotherapeutic microcapsules).