Figures & data
Figure 1. Proposed cross-talk between EpCAM signaling and the Wnt pathway. Activation of the frizzled receptor by members of the Wnt family of ligands induces the inhibition of GSK3β and the subsequent stabilization of β-catenin. Upon nuclear translocation, β-catenin controls Lef-1 dependent transcription. EpICD interacts with the very same components to form a nuclear complex comprised of β-catenin, FHL2 and Lef-1. This nuclear complex is licensed to bind promoters of genes involved in cell cycle regulation and stemness. Further research would be necessary to describe in-depth the mechanism of transcriptional regulation of Nanog, Oct4, Klf4 and Sox2 by EpCAM. The actual composition of EpICD nuclear complexes recruited to the promoters of stemness genes are unexplored until now.
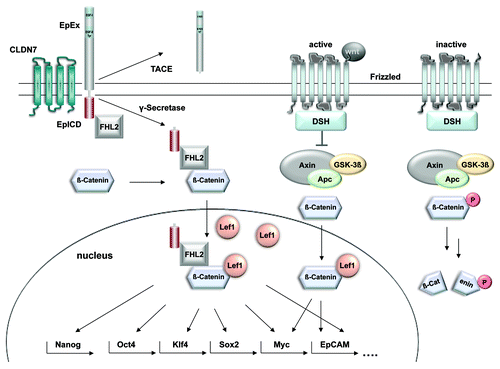
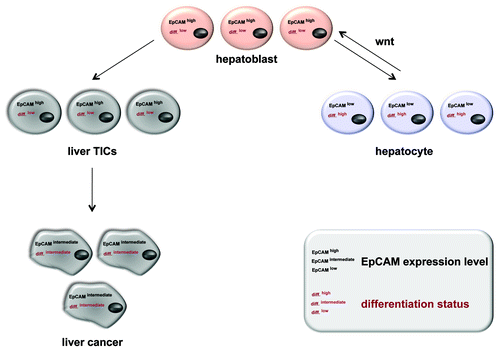
Figure 2. EpCAM expression in normal and regenerating liver, mature hepatocytes, liver carcinomas and TICs thereof. During liver morphogenesis and regeneration upon injury or chronic inflammation, EpCAM expression correlates with a proliferative and low differentiation. Liver precursors (hepatoblasts) are characterized by EpCAMhigh and downregulate this EpCAM expression upon differentiation into mature hepatocytes (differentiationlow). This process is partly regulated by the Wnt signaling pathway, which also dictates EpCAM expression. For the case of malignant transformation, EpCAM remains highly expressed in liver TICs and show an intermediate phenotype in hepatocellular carcinomas (differentiationintermediate).