Figures & data
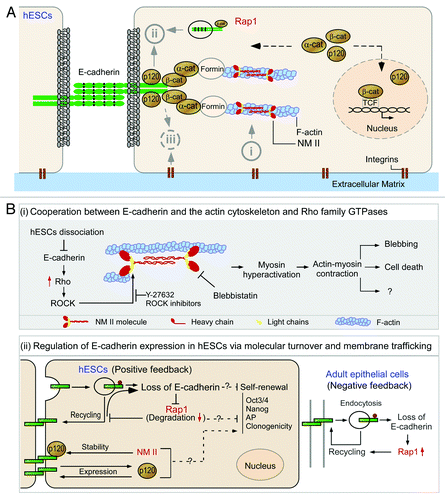
Figure 1. E-cadherin and its associated molecules in the survival and differentiation of human embryonic stem cells. (A) The molecular structure and the binding sites of E-cadherin and its connection with actin cytoskeleton. The extracellular region of E-cadherin consists of five cadherin-type repeats (extracellular cadherin domains) that are bound together by Ca2+ ions. The cytoplasmic tails of E-cadherin bind to p120-catenin (p120) and β-catenin (β-cat), while β-catenin binds to monomeric α-catenin (α-cat) which indirectly anchors the cadherin-catenin complexes to the actin filaments (F-actin). NM II molecules assemble into bipolar filaments by interactions between their rod domains. These filaments bind to actin through their head domains where the ATPase activity enables a conformational change that moves actin filaments in an anti-parallel manner. (B) Schematic view of the pathways associated with E-cadherin in the survival of human embryonic stem cells. (i) Cooperation between E-cadherin and the actin cytoskeleton and Rho family GTPases in the regulation of survival of dissociated human embryonic stem cells (hESCs). The subunit of non-muscle myosin II (NM II) forms a dimer through interactions between the α-helical coiled-coil rod domains. The globular head domain contains the actin-binding regions and the enzymatic Mg2+-ATPase motor domains. The light chains bind to the heavy chains at the lever arms that link the globular head and rod domains. Dissociation of hESCs results in disruption of E-cadherin mediated cell-cell adhesions and subsequently leads to Rho/ROCK activation and phosphorylation of myosin light chain. Y-27632 inhibits ROCK activation and therefore suppresses the phosphorylation of myosin light chain and promotes the survival and cloning efficiency of hESCs. Blebbistatin inhibit heavy chains and facilitate survival of dissociated hESCs. (ii) Regulation of E-cadherin expression in hESCs via molecular turnover and membrane trafficking. Unlike epithelial cells, hESCs lack a negative feedback-like mechanism between Rap1 and E-cadherin. After disassembly of adherens junctions, Rap1 is delivered to lysosomes and degraded, which further hampers hESC clonogenicity. There is another unique positive feedback loop between p120-catenin (p120) and E-cadherin in hESCs. Unlike epithelial cells, E-cadherin increases p120 production, while p120 boosts E-cadherin expression. p120 seems to facilitate E-cadherin accumulation at cell-cell junctions, thereby enhancing the self-renewal of hESCs.