Figures & data
Figure 1. Cell migration phenotype on 2D surface and in 3D matrix. While migrating cells detach frequently from neighboring cells on a collagen-coated 2D surface, migrating cells maintain cell-cell contacts in a 3D collagen matrix. The cells are hepatocyte growth factor (HGF)-treated MDCK epithelial cells that have undergone a complete EMT. White arrowhead tracks the position of a migrating cell. Scale bar, 20 μm. Time in minutes.
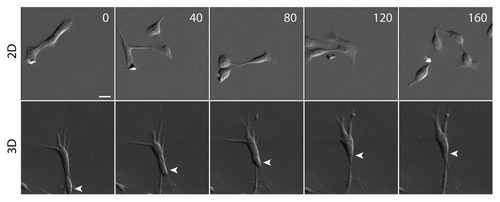
Figure 2. N-cadherin-mediated cell-cell adhesion regulates collective cell migration. (A) Cell clusters adopt different migration phenotypes depending on the strength of N-cadherin mediated cell-cell adhesion. In the absence of N-cadherin-mediated cell-cell adhesion (e.g., the extracellular domain deleted N-cadherin mutant expressing cells), the cells are migratory but remain single cells. With weak cell-cell adhesion (e.g., the cytoplasmic deleted N-cadherin mutant cells), migratory cells often form cell clusters but cell-cell interactions are transient. In wildtype cells, cell-cell adhesion is strong enough to minimize cell scattering, but dynamic enough to support cell intercalation within cell clusters. The expression of an N-cadherin-α-catenin chimera induces strong cell-cell adhesion that prevents cell intercalation, but promotes supracellular movement of the entire cluster. (B) Model depicting different pathways through which N-cadherin can regulate cell migration. N-cadherin 4th extracellular domain has been shown to interact with fibroblast growth factor receptor (FGF receptor), which is involved in the pro-migratory MAPK/ERK pathway. N-cadherin cytoplasmic domain interacts with binding partners such as β-catenin (β-cat), which interacts with α-catenin (α-cat), a molecule that can bind and organize the actin network. Furthermore, N-cadherin cytoplasmic domain may be regulating cell migration through the regulation of GTPase activity. N-cadherin binding partner, β-catenin also binds RICS, a GTPase activating protein for cdc42 and Rac1. Or, N-cadherin binding partner, p120-catenin (p120-cat) binds p190RhoGAP (p190), which is a GTPase activating protein for RhoA.
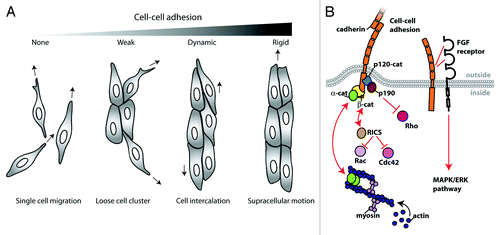
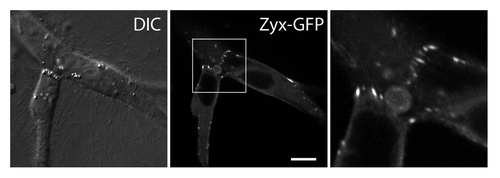
Figure 3. Zyxin accumulates at sites of force-bearing N-cadherin mediated cell-cell junctions in transformed epithelial cells migrating in a 3D matrix. Zyxin-GFP expressing MDCK cells are embedded in a collagen gel, and imaged using a confocal microscope. Zyxin accumulates as focal adhesion-like puncta at cell-cell contacts in a 3D matrix. Scale bar, 10 μm.