Figures & data
Figure 1. Cell rounding by CD34 in HEK293T cells. (A) Phase contrast images of HEK293T cells transfected with empty vector (HEK293T cells) or a CD34 expression vector (CD34-HEK293T cells). CD34-HEK293T cells were rounded and detached from the substrata (b), whereas HEK293T cells were flat (a) at 40 h after transfection. Scale bar: 100 µm. (B) Fluorescence images of HEK293T cells at 2 d after transfection of a GFP expression vector (a) or a GFP expression vector plus a CD34 expression vector (b). Scale bar: 100 µm. (C) Ratio of round cells among GFP+ cells shown in (B).
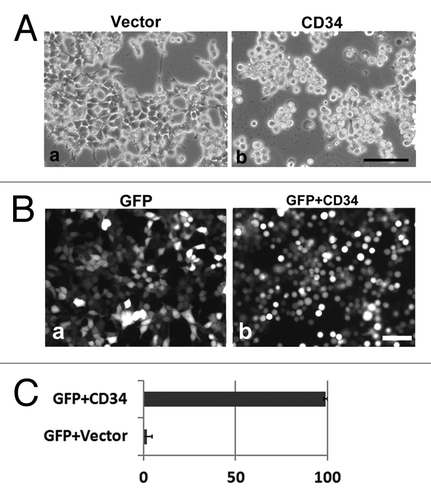
Figure 2. Formation of microvilli-like protrusions in CD34-HEK293T cells. (A) Subcellular localization of CD34-GFP in HEK293T cells. Images of HEK293T cells transfected with a CD34-GFP expression vector (CD34GFP-HEK293T cells) were captured at low magnification on a culture dish (a) or at high magnification on a glass coverslip (b). Scale bars: 100 µm (a) and 10 µm (b). (B) Subcellular localization of CD34 in CD34-HEK293T cells. CD34-HEK293T cells were double stained with anti-CD34 (a) or anti-Ezrin (d) antibodies and phalloidin (b and e). Staining was co-localized in microvilli-like protrusions from CD34-HEK293T cells. Merged images (c and f). Scale bars: 10 µm. (C) Electron microscopy of HEK293T transfectants. Scanning electron micrograph of Vector-HEK293T (a) and CD34-HEK293T (b) cells. Ultrathin section electron micrograph of Vector-HEK293T (c) and CD34-HEK293T (d) cells. Magnified images of microvilli-like protrusions from Vector-HEK293T (e) and CD34-HEK293T (f) cells. Arrowheads indicate lipid bilayer membranes. Arrows indicate actin filaments. Scale bars: 5 µm (a and b) and 500 nm (c–f). (D) Immunoblot analysis of ERM proteins in CD34-HEK293T cells. Alterations of ERM phosphorylation was detected by immunoblotting. (E) Immunofluorescence with an anti-p-ERM antibody. p-ERM proteins were observed at microvilli-like protrusions from CD34-HEK293T cells (b). Scale bars: 10 µm.
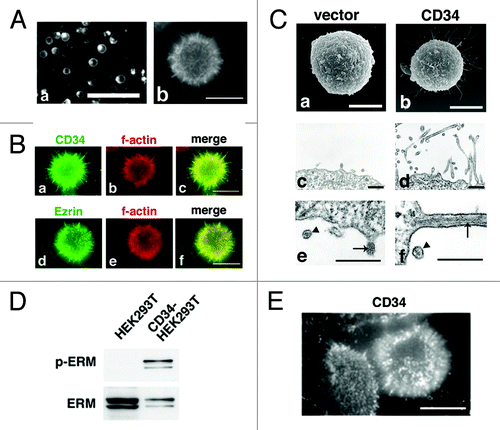
Table 1. Table of the length and number of microvilli
Figure 3. Inhibition of integrin-mediated cell adhesion by CD34. (A) Effect of CD34 on integrin-mediated re-attachment of α4-HEK293T cells. α4-HEK293T cells were transfected with a GFP expression vector (GFP-α4-HEK293T cells) or a CD34-GFP expression vector (CD34-GFP-α4-HEK293T cells). Cells were harvested, re-plated, and incubated in GST-CS1-coated wells, and then washed with medium. Adherent cells were evaluated by fluorescence microscopy. Scale bar: 200 µm. (B) Comparison of the number of bound cells. The average numbers of adherent cells with even weak fluorescence was calculated from three experiments. The histogram indicates the average numbers of adherent fluorescent cells on the coated surface. GFP-α4-HEK293T cells, CD34-GFP-α4-HEK293T cells, GST, and GST-CS1 are indicated as G, 34G, GST, and CS1, respectively. (C) Flow cytometric analysis of GFP in bound and unbound cells. The fluorescent intensity of GFP and CD34-GFP in cells bound (dashed line) or unbound (continuous line) to the GST-CS-1-coated substrata is shown. Cells prior to the binding assay are indicated by the shaded areas. The expression level of CD34-GFP in unbound CD34-GFP-α4-HEK293T cells in GST-CS1-coated wells was higher than that in bound cells.
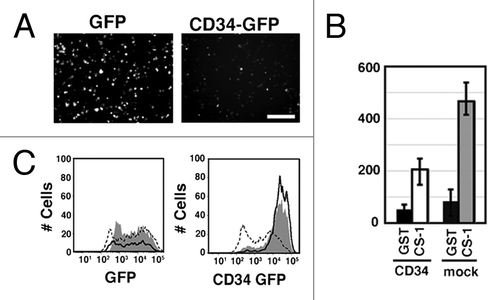
Figure 4. Microvilli in human CD34+ HPCs. (A) SEM of a CD34+ bone marrow cell (a) and CD34+ cord blood cell (b). Scale bars: 2 µm. (B) Immunofluorescence of a CD34+ bone marrow cell (a–c) and a CD34+ cord blood cell (d–f). CD34+ cells were double stained with an anti-CD34 antibody (a and d) and phalloidin (b and e). Merged images (c and f). Scale bars: 5 µm.
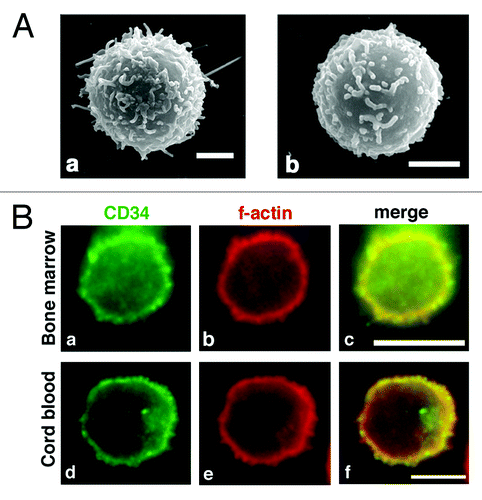
Figure 5. CD34 is localized at microvilli in KG-1 cells. (A) Electron microscopy of KG-1 cells. Scanning electron micrograph (a) and ultrathin section electron micrograph (b) of KG-1 cells. KG-1 cells were covered with numerous microvilli. Arrows indicate parallel filamentous actin. Scale bars: 2 µm (a), 500 nm (b), and 125 nm (inset). (B) Subcellular localization of CD34 in KG-1 cells. KG-1 cells were double stained with anti-CD34 (a), anti-ezrin (d), or anti-β1-integrin antibody (g) and phalloidin (b, e, and h). Merged images (c, f, and i). Scale bars: 10 µm.
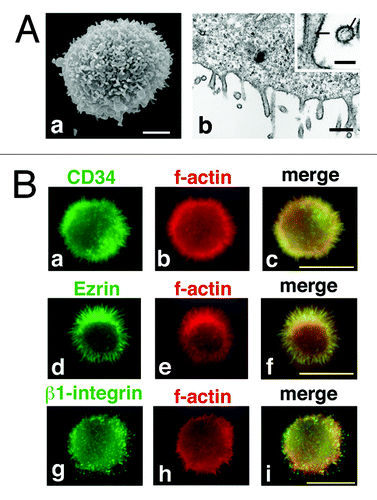
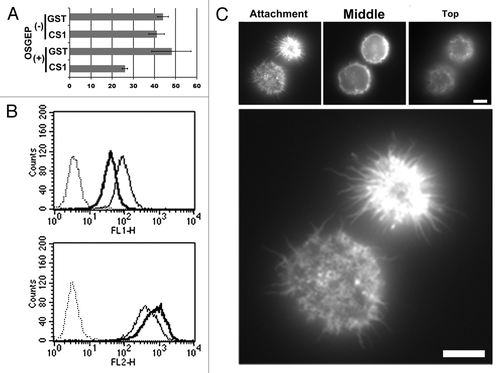
Figure 6. Effect of OSGEPase treatment on integrin-mediated adhesion of KG-1 cells. (A) Numbers of unattached KG-1 cells in GST-CS1-coated wells. KG-1 cells were treated with or without OSGEPase for 30 min. Cells were incubated in tissue culture plates coated with GST or GST-CS1, washed, and then the numbers of unattached cells were counted. (B) Flow cytometric analysis of OSGEPase-treated and untreated KG-1 cells. Cells were double immunostained with fluorophore-labeled anti-CD34 antibodies, 581 (PE) and QBEnd10 (FITC). OSGEPase treatment reduced QBEnd10 on KG-1 cells. (C) Phalloidin staining of KG-1 cells. OSGEPase-treated KG-1 cells were attached to GST-CS-1-coated glass chamber, fixed, permeabilized, and then stained with phalloidin. Images of attachment, middle, and top phases are shown. Scale bar: 1.25 μm.