Figures & data
Figure 1 Fibronectin accelerates cell attachment and spreading. IEC-6 cells were grown to confluence in DMEM containing 5% dialyzed FBS for 3 days followed by serum starvation for 24 h. The cells were trypsinized, counted, conditioned and equal numbers were seeded on plastic- and fibronectin (FN)-coated plates in DMEM without serum. (A) Upper: phase contrast pictures 4 h post plating. Lower: Cells were fixed and stained for F-actin. (B) One set of plates was washed with HBSS, trypsinized and the attached cells were counted. Cell numbers plotted with respect to the area of the plate. Values are mean ± SEM of triplicates. *Significantly different (p < 0.05). (C) Equal amounts of protein were separated using SDS-PAGE for western blot analysis. Blots were probed with specific antibodies as shown in the figure. Actin was used as loading control. Representative blots from three observations are shown. (D) Cell migration assay was carried out as described in the methods and phase contrast pictures taken at 0 and 7 h post wounding are shown. (E) IEC-6 cells grown as described above were trypsinized, counted and equal numbers were seeded on plastic and fibronectin coated plates in DMEM in the presence of 5% dialyzed FBS. Phase contrast pictures at 4 h post plating are shown. Rac-1 activity (GTP-Rac1) was determined by GST-PAK pull down assay. Representative blots for the total- and active-Rac1 levels from three observations are shown.
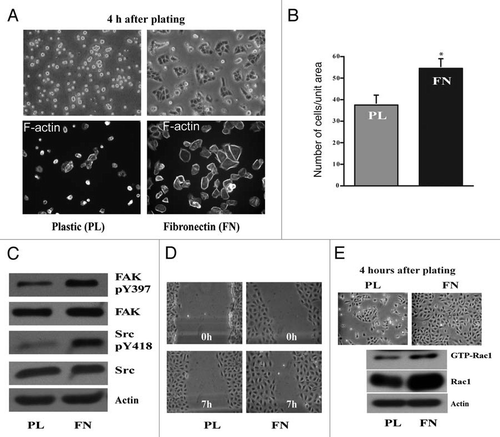
Figure 2 RGDS inhibits migration. IEC-6 cells were grown to confluence in control, DFMO and DFMO plus putrescine containing media for 3 days followed by serum starvation for 24 h. Confluent monolayers were wounded with a gel loading tip in the center of the plates, washed and left untreated or treated with 1 mM RGDS. (A) Plates were photographed immediately to record the wound width (0 h) and again at the marked wound location after 7 h. (B) Wound area covered during migration was calculated as described in the methods. Values are mean ± SEM of triplicates. *Significantly different compared to respective untreated samples. (C) IEC-6 cells were grown to confluence in control medium for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned for 30 min at room temperature before plating on plastic and fibronectin-coated plates in the presence or absence of RGDS (1 mM). Cell lysates were analyzed for pY397FAK, total FAK, and active Rac1 by SDS-PAGE. Actin served as loading control. (D) IEC-6 cells were grown to confluence in control medium for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned for 30 min at room temperature before plating on plastic and fibronectin-coated plates in the presence or absence of RGDS (1 µM) and allowed to attach for about 3 h in serum free medium. Equal amounts of cell lysate immunoprecipitated using Tiam1 antibody were probed with phosphotyrosine (pY99) and Tiam1 antibodies.
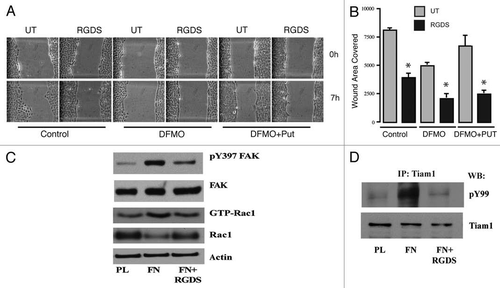
Figure 3 Polyamine depletion delays cell attachment and spreading. IEC-6 cells were grown to confluence in control and DFMO containing media for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned in medium with or without DFMO for 30 min at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at time 0. (A) Phase contrast images taken at different time points during attachment and spreading are shown (B) 20 µg protein from each sample was analyzed by western blotting to detect phosphorylated Src and FAK. Actin was used as loading control. Densitometric analysis of western blots was carried out using NIH image J software. The ratio of the phosphorylated versus total protein (specific activity) for FAK (C) and Src (D) from 3 experiments is shown. Values are mean ± SEM of triplicates. *Significantly different compared to respective control (p < 0.05).
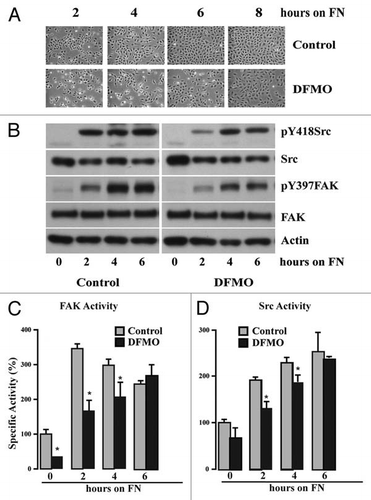
Figure 4 Polyamine depletion decreases Tiam1 protein and Rac1 activity. IEC-6 cells were grown to confluence in control and DFMO containing media for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned in medium with or without DFMO for 30 minutes at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at time 0. (A) 20 µg protein from each sample was analyzed by western blotting to detect Tiam1 and pThr-Tiam1 proteins. (B) Densitometric analysis of Tiam1 levels from 3 different experiments. Values are mean ± SEM of triplicates. *Significantly different compared to respective control (p < 0.05). (C) GTP-Rac1 separated using GST-PAK pull down as described in the methods. The western blots were scanned and the specific activity of Rac1 was calculated as a ratio of GTP-Rac1 (active) to the total Rac1. A representative experiment for the pattern of Rac1 activity is shown. (D) Cells were grown to confluence in control, DFMO and DFMO plus putrescine containing media for 3 days and serum starved for 24 h, trypsinized and plated on fibronectin-coated plates and allowed to attach for 4 h. Attached cells were lysed and 20 µg protein from each sample was analyzed by SDS-PAGE using specific antibodies. Representative blots from three observations are shown.
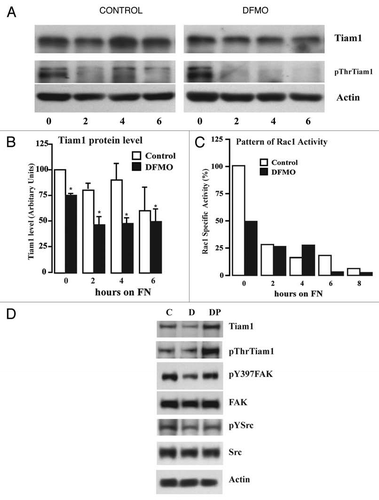
Figure 5 Inhibition of Tiam1-Rac1 binding abrogates Rac1 and FAK activity. IEC-6 cells were grown to confluence for 3 days and serum starved for 24 h. Cells were trypsinized, counted and conditioned in medium with 5% dialyzed FBS for 30 min at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at 0 h. Cells were allowed to attach in the presence and absence of NSC23766 (120 µM) and lysed at timed intervals. (A) Western blot after GTP-Rac1 pull down (B) 20 µg protein was separated on SDS-PAGE and analyzed by western blotting using specific antibodies. (C) Migration studies were carried out as described in methods with or without NSC23766. Values are mean ± SEM of triplicates. *Significantly different compared to untreated (p < 0.05). Representative blots from three observations are shown.
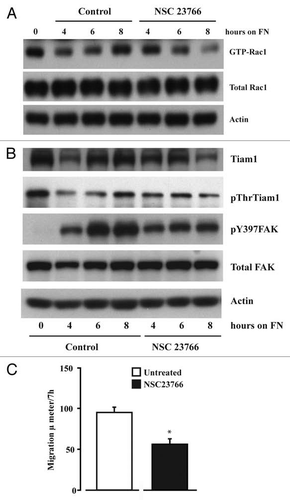
Figure 6 Src activity is required for cell adhesion and migration. IEC-6 cells were grown to confluence for 3 days and serum starved for 24 h. Cells were trypsinized, counted, and conditioned in medium with 5% dialyzed FBS for 30 min at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at 0 h (C-0). Conditioned cells were plated in the presence of 5 or 10 µM PP 2 or DMSO (vehicle). (A) Images were captured at 0.5 and 1.0 h. (B) The cells were washed gently and attached cells were trypsinized and counted using the coulter counter. Values are mean ± SEM of triplicates. *Significantly different compared to vehicle (V) DMSO (p < 0.05). (C) Cells were lysed after 4 h and 20 µg protein was used for western blot analysis of pY418 Src, total Src, pY397FAK and total FAK. (D) Western blots for pAKT, Tiam1, GTP-Rac1, and total Rac1. Actin was used as loading control. (E) Migration studies carried out as described in methods with or without PP 2 (10 µM). Values are mean ± SEM of triplicates. *Significantly different compared to vehicle (V) DMSO (p < 0.05). (F) IEC-6 cells plated on plastic and fibronectin-coated plates with or without PP 2 (10 µM) for 4 h were lysed and 20 µg protein was analyzed by western blotting for pY397FAK, pY118Paxillin, and vinculin using specific antibodies. Representative blots from three observations are shown.
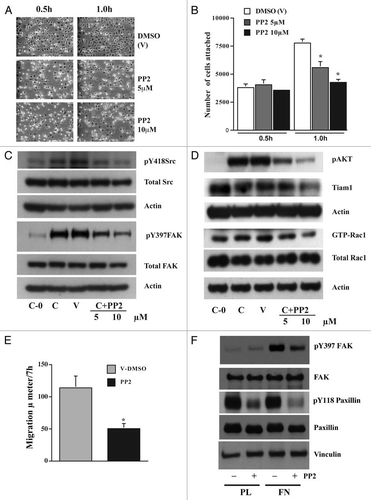
Figure 7 FAK modulates Tiam1. IEC-6 cells were grown to confluence for 3 days and serum starved for 24 h. Conditioned cells were seeded on fibronectin-coated plates with or without the focal adhesion kinase inhibitor FAK-14 (10 µM). (A) Cells were photographed after 45 min and lysed. (B and C) 20 µg protein was separated on SDS-PAGE and analyzed by western blotting using specific antibodies specific antibodies. GTP-Rac1 levels were determined by pull down assay as described in methods. Representative blots from three observations are shown.
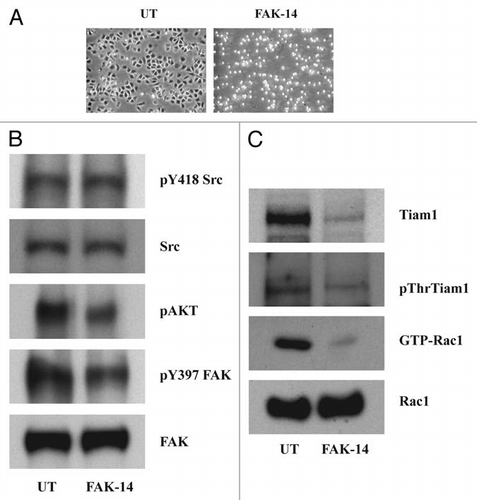
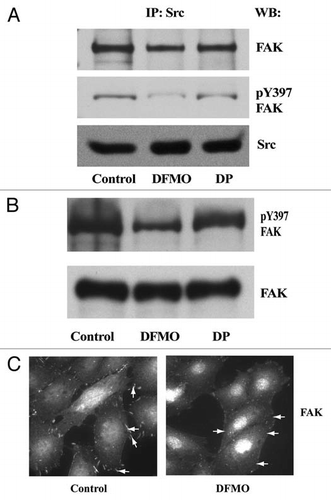
Figure 8 Src and FAK associate at the focal adhesions. IEC-6 cells grown in control, DFMO and DFMO plus putrescine containing media for 3 days, serum starved for 24 h were washed and lysed. (A) Src was immunoprecipitated from these lysates and FAK/pY397FAK bound to it was detected by immunoblotting with specific antibodies. (B) 20 µg protein from whole cell lysate separated on SDS-PAGE was subjected to western blot analysis using pY397FAK and total FAK antibodies. Representative blots from three observations are shown. (C) Cells grown in control and DFMO media were plated on glass cover slips and immuno-stained for FAK. Representative images from three experiments are shown.