Figures & data
Figure 1 Analysis of proteins associating with ZF21. (A) ZF21 fused to GST was used to pull down proteins from whole cell lysates of HeLa cells as described previously.Citation9 Proteins specifically bound to GST-ZF21 were analyzed by western blot using antibodies against the indicated proteins (FAK, talin, vinculin or GST). (B) The proteins pulled down using GST-ZF21 or GST-EGFP were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue R-250. The protein bands that appeared to bind specifically to GST-ZF21 but not to GST-EGFP were cut out and these bands were subjected to in-gel digestion with trypsin and analyzed by LC/MS. Controls were the corresponding gel fragments in GST-EGFP column. Asterisks indicate GST-EGFP and GST-ZF21 proteins.
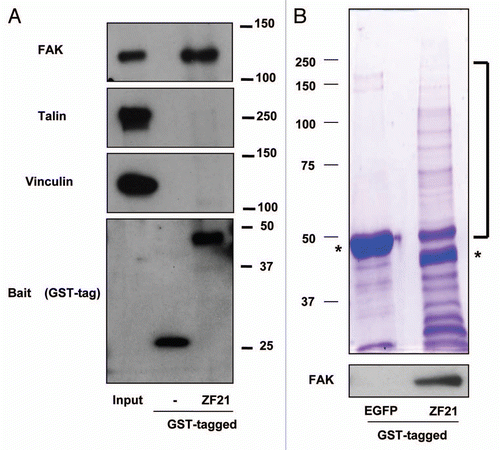
Figure 2 A possible mechanism of ZF21-mediated disassembly of FAs. (A) Domain structure of GST-ZF21 derivatives used for the pull-down assays in (B). Full (1–234 aa), full-length; N (1–105 aa), an N-terminal domain fragment including FYVE domain; C (106–234 aa), a C-terminal domain mutant. The amino acid positions are indicated by aa. (B) Pull-down experiments were carried out as described in . The proteins bound to GST-ZF21 derivatives were subjected to western blot analysis using antibodies against FAK, m-calpain, b-tubulin or GST. (C) Binding regions of ZF21 with FAK, m-calpain or b-tubulin are summarized based on the results in (B). (D) A model proposed for ZF21-mediated disassembly of FAs, though association of proteins with ZF21 may not be direct. ZF21 resides in FAs even when MT extension to the FA is disrupted by nocodazole treatment. ZF21 binds to FAK in FAs via its FYVE domain. The C-terminal region of the pre-existing ZF21 in FAs may bind MTs that have extended to the FAs. ZF21 may also reside in the transport vesicles via the FYVE domain. Since ZF21 can bind SHP-2 and m-calpain, these factors might also be transported to FAs by MTs if ZF21 interacts with the vesicles transported by kinesin-1 on MTs. Binding of m-calpain to ZF21 requires the entire region of the protein, but the binding site of ZF21 to SHP-2 is not known. The pre-existing ZF21 in FAs may form oligomers with the ZF21 transported to the FAs by vesicles. The ability of ZF21 to form oligomers in FAs may bring phosphorylated FAK in closer proximity to SHP-2. m-Calpain cleaves integrin, FAK, talin, paxillin and vinculin. The ZF21 oligomer also acts as a platform to accumulate factors for executing the disassembly of FAs. Thus, MT extension to FAs causes accumulation of factors responsible for the initiation of the disassembly of FAs. Finally, the remains of the disassembled FAs are internalized in a dynamin-dependent manner.
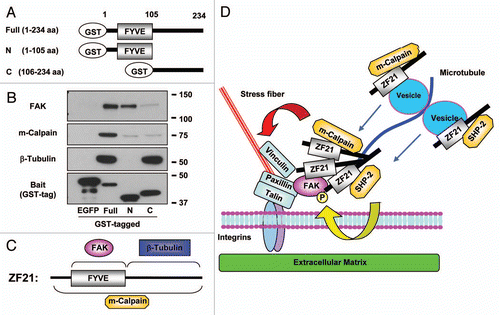
Table 1 List of ZF21-binding proteins identified in