Figures & data
Figure 1 The accumulation of Cav1 at peripheral FAs following Rac1 activation requires Src activity. Hela cells were grown on glass coverl slips and stimulated as indicated with CNF1 (500 ng/ml, 4 h) (Co) localization of endogenous Cav1 with endogenous paxillin was analyzed by immunostainings in control, CNF-1-treated or CNF-1-treated Hela cells that were also treated with PP2 (10 µM) for 30 min prior to fixation. Cav1-paxillin co-localization resulting from the activation of endogenous Rac1 by CNF1 is indicated by the arrows. (Scale bar, 20 µm).
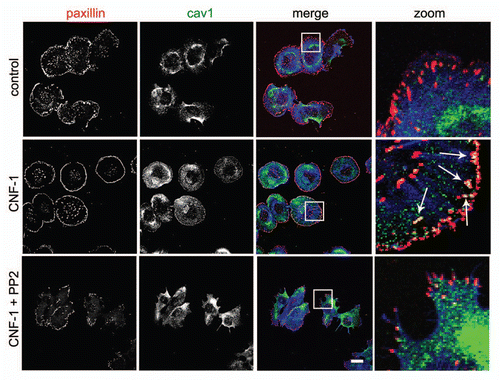
Figure 2 Src cooperates with activated Rac1 in the recruitment of Cav1 to FAs. (A) SYF (-/-) MEFs were grown on glass cover slips and transfected with myc-tagged Rac1 (Q61L). After 24 h, cells were fixed and stained for (co)localization of Rac1Q61L and endogenous paxillin as indicated by the arrows (scale bar, 20 µm). (B) The (co)localization of myc-tagged Rac1 (Q61L) with endogenous Cav1 was analyzed by immunostainings in SYF(-/-) MEFs and Src knock-in SYF (-/-) MEFs (SYF-/- + Src MEFs). In the SYF-/- cells, there is noo co-localization detectable between peripheral Rac1 and Cav1. In contrast, in the Src reconstituted cells, colocalization between Rac1 and Cav1 at the periphery of the cells is detectable and these are indicated by the arrows. (Scale bar, 20 µm).
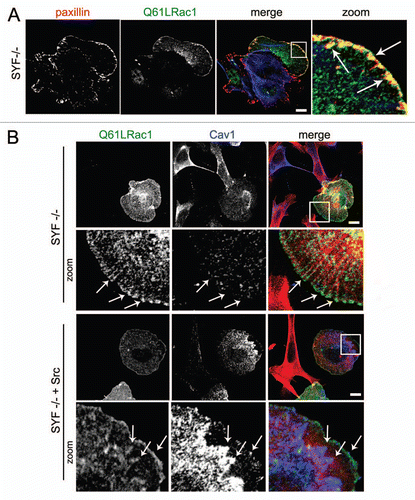
Figure 3 Caveolin1-positive vesicles colocalize with early endosomes in the cellular periphery. HeLa cells were grown on glass cover slips and transfected with Rac1 (Q61L) and GFP-2xFYVE as a marker for early endosomes. After 24 h, cells were fixed and stained for (co)localization of Rac1 and Cav1. Early endosomes were identified by GFP imaging. Arrows indicate vesicular structures positive for Rac1 and Cav1, a fraction of which colocalize. (Scale bar, 10 µm).
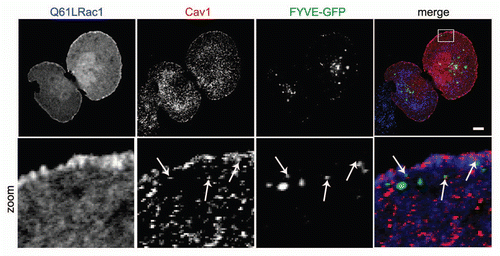
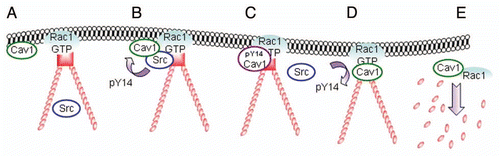
Figure 4 Model for the translocation, phosphorylation and dissociation of Cav1 at FAs. (A) Active Rac1 promotes the formation of FAs and promotes the recruitment of Cav1 to the cellular periphery. (B and C) subsequent association of Cav1-positive vesicles with Src on endosomes drives the phosphorylation of Cav1 and the accumulation of pY14Cav1 at FAs. (D and E) A FA-associated tyrosine phosphatase dephosphorylates Cav1 which stimulates the internalization of Cav1 and associated FA-components. This is concomittant with FA turnover which is required for efficient cell migration.