Figures & data
Figure 1 Src-induced formation of podosomes and rosettes in rat aortic smooth muscle cells that actively digest extracellular matrix. (A) A control rat aortic smooth muscle cell (RASMC) showing prominent actin-stress fibers stained with FITC-phalloidin. (B) RASMC line expressing constitutively active Src(Y527F) encoded in retroviral vectors. Note the disappearance of actin-stress fibers and formation of podosomes that self-assemble into rosettes. (C) Digestion of TRITC-labeled fibronectin (red) in ECM by rosettes appears as dark spots. Actin is stained with FITC-phalloidin (green). Scales bars represent 20 µm.
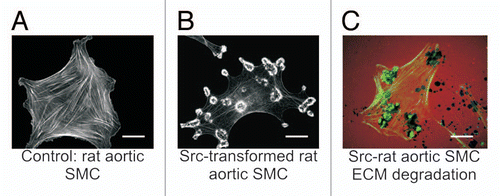
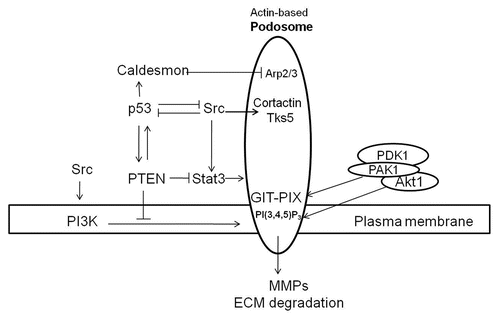
Figure 2 Proposed mechanisms for anti- and pro-podosome formation mediated by p53 and Src, respectively. p53 suppresses podosome formation in VSMC by upregulating caldesmon and PTEN. Caldesmon acts as a podosome antagonist by inhibiting Arp2/3-mediated actin polymerization. Both PTEN protein- and lipid-phosphatase activities contribute to podosome suppression by downregulating the Src-Stat3 and Src-PI3K-Akt pro-podosome and invasion pathways. Membrane receptor tyrosine kinases activate Src, which induces podosome formation and cell invasion by phosphorylating podosomal proteins such as cortactin and Tks5/FISH. Activation of PI3K by receptor tyrosine kinases or Src increases PI(3,4,5)P3, which together with GIX-PIX recruit the Akt-PAK1-PDK1 ternary complex to sites of podosome biogenesis at the plasma membrane. Activated Akt and PAK1 proceed to modulate regulatory proteins engaged in podosome dynamics. While p53 and Src mutually antagonize each other, a positive feedback loop appears to exist between p53 and PTEN.