Figures & data
Figure 1 The inhibition of the progression of breast cancer growth by SU11248 in the immunocompetent female mice (C57BL/6) allografted with mouse breast cancer (E0771) cells. SU11248 treatment at 20 to 40 mg/kg/d in drinking (distilled) water significantly reduced a growth curve of breast cancer monitored by the tumor cross section area (A, p < 0.01; n = 8), tumor weight (B, p < 0.01) and tumor size (C, p < 0.01), compared to the control group.
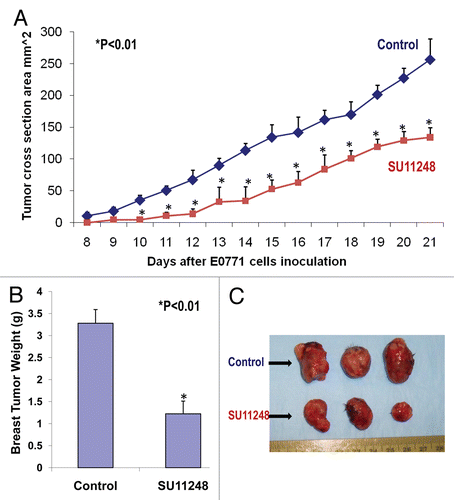
Figure 2 The digital images of CD31 immunohistochemistry staining in OCT-embedded cryosections of mouse breast cancer tumors obtained from the control and SU11248-treated mice (A). Sections were incubated with rat anti-mouse CD31 antibody followed by mouse antirat IgG (Vector laboratories, Burlingame, CA), Extravadin Peroxidase (Sigma, St. Louis, MO) and peroxidase substrate (Vector laboratories, Burlingame, CA). Sections were counterstained with hemotoxylin. The brown staining indicated microvascular vessels. Morphometric analysis (B) indicated that SU11248 treatment caused a significant decrease in average microvessel density (AMVD, the number of microvessels per mm2 area) of breast cancer tumors compared to the control breast cancer tumors (155 ± 6 vs. 111 ± 10 microvessels number per mm2; n = 8; p < 0.01).
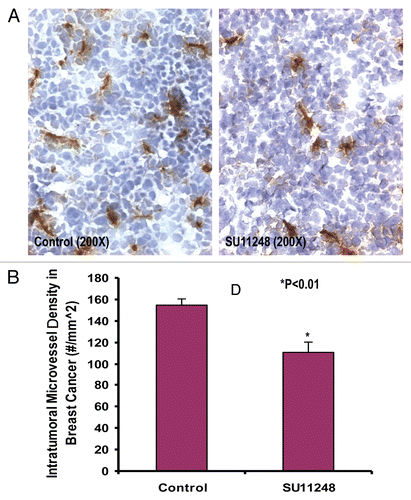
Figure 3 The mRNA expression of mouse VEGF receptor-1 (Flt-1) and VEGF receptor-2 (Flk-1) in cultured E0771 cells by reverse transcriptase-PCR analysis. (A) shows Flt-1 bands (115 bp). (B) shows Flk-1 bands (115 bp).
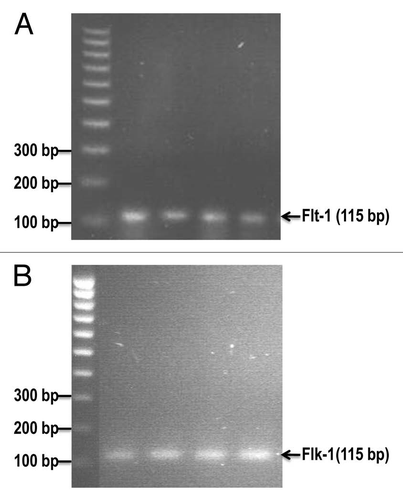
Figure 4 Effects of VEGF or VEGF + SU11248 on the proliferation of cultured mouse breast cancer (E0771) cells, human umbilical vein endothelial cells (HUVEC) and human aortic smooth muscle cells (HAS MC). VEGF (10 ng/ml) caused a 42% increase in the proliferation of E0771 cells, compared to the control (p < 0.01; n = 8) and there was a significant decrease in the proliferation of E0771 cells treated with VEGF plus SU11248 vs. the control (65%, p < 0.01) (A). VEGF caused a 2-fold increase in the proliferation of HUVEC vs. the control (p < 0.01; n = 8), but its action was completely abolished by SU11248 (B). Neither VEGF nor SU11248 exerted any effect on the proliferation of cultured HAS MC (1) that do not express VEGF receptors (C).
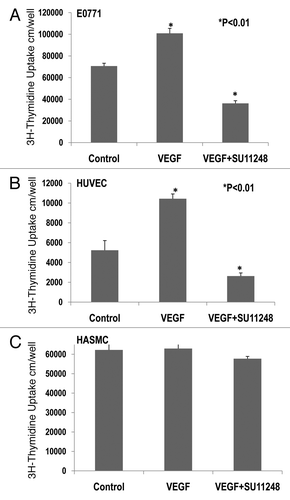
Figure 5 The light microscope images of the invaded E0771 cells on the outside of Matrigel inserts (A: Control; B: SU11248). VEGF receptor inhibitor, SU11248 at 10 µmol/L, significantly inhibited the migration of mouse breast cancer cells (E0771) in Matrigel by 47%, compared to the control group (61 ± 3 vs. 32 ± 2 invaded cell numbers/mm2; p < 0.01; n = 8).
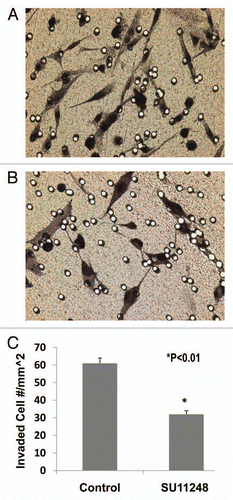
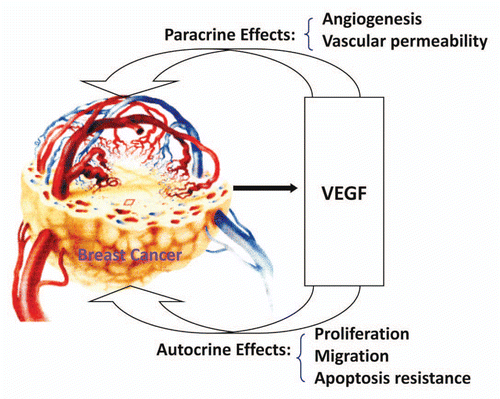
Figure 6 The expression of VEGF and VEGFR1 in cultured ER-positive (MCF-7) and ERnegative (MDA-MB-231) human breast cancer cells. ELISA assay showed that VEGF protein was expressed much more in ER-negative (MDA-MB-231) human breast cancer cells than in ER-positive (MCF-7) human breast cancer cells (>8-fold; p < 0.01; n = 8). Also, VEGF receptor-1 (flt-1) protein was more highly expressed in ER-negative (MDA-MB-231) human breast cancer cells than ER-positive (MCF-7) human breast cancer cells (>7-fold; p < 0.01; n = 8).
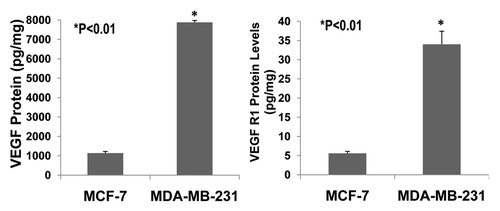
Figure 7 Effects of 17β-estradiol on the expression of VEGF protein in cultured ER-positive (MCF-7) and ER-negative (MDA-MB-231) human breast cancer cells. ELISA assay showed that 17 β-estradiol (5 nmol/L) significantly increased VEGF expression in cultured ER-positive (MCF-7) human breast cancer cells (1,762 ± 56 vs. 1,136 ± 58 pg/mg; p < 0.01; n = 8) compared to the control group, but not in ER-negative (MDA-MB-231) human breast cancer cells.
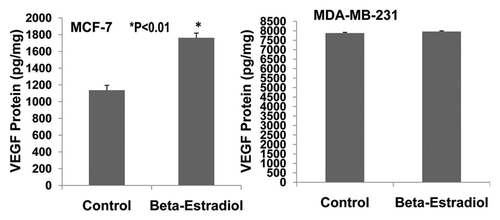
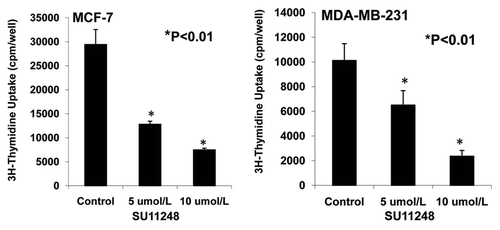
Figure 8 Effects of SU11248 on the proliferation of cultured ER-positive (MCF-7) and ER-negative (MDA-MB-231) human breast cancer cells. 3H-thymidine incorporation showed that 5 and 10 µmol/L of SU11248 caused a 56% and 77% decrease in the proliferations of ER-positive (MCF-7) human breast cancer cells, respectively; in contrast to the control (p < 0.01; n = 8). 5 and 10 µmol/L of SU11248 caused a 36% and 75% decrease in the proliferations of ER-negative (MDA-MB-231) human breast cancer cells, respectively; in contrast to the control (p < 0.01; n = 8).