Figures & data
Figure 1 ERG regulates HPGD expression and PGE2 in VCaP cells. (A) VCaP cells transfected with ERG siRNA (E1, E2) or with non-targeting siRNa (NT) from triplicate experiments were harvested on day 4 post transfection and processed for immunoblot analysis for detecting ERG and HPGD. Tubulin expression was used as the input control. (B) Induction of HPGD expression in response to ERG knockdown in a time dependent fashion. Transfected VCaP cells, as described in the Materials and Methods, were harvested on days 1, 4 and 8 post-transfection and processed for immunoblot assay for detecting ERG and HPGD proteins. (C) Ectopic expression of ERG decreased HPGD protein levels. VCaP cells were infected with either wild type ERG2 adenovirus expression vector (Adv-ERG2) or control adenovirus expression vector (Adv-CTL). (D) Sub-cellular localization of ERG and HPGD in response to ERG siRNa (E1) or non-targeting siRNA (NT) was assessed by immunofluorescence assay in VCaP cells. (E) PGE2 levels were measured in the conditioned medium of VCaP cells transfected with either non-targeting siRNA (NT) or ERG siRNA (E1) in the presence of IL-1β. Cells were harvested after 24 h and total lysate were prepared for PGE2 normalization (insert).
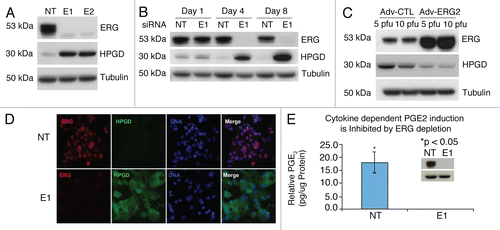
Figure 2 ERG is recruited to the HPGD core promoter ETS binding site in VCaP cells. ERG recruitment is specific to the core ETS binding site of HPGD and is eliminated by ERG siRNA treatment. Upstream and downstream sequences with no ETS binding element were used as negative controls. In the ChIP assay recruitment of ERG to the KLK3/PSA, C-MYC and SLC45A3 gene promoter upstream regions were also tested as positive controls of ERG binding as similar data have been reported before (controls).Citation5 Input indicates control genomic DNA amplicons.
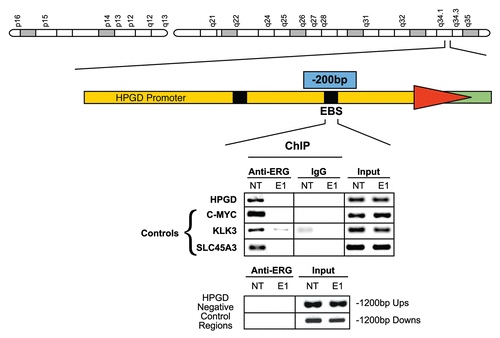
Figure 3 PGE2 induced CaP cell growth is inhibited by ERG knockdown. VCaP cells were evaluated for cell growth in the presence (+) or absence (−) of PGE2 in response to non-targeting siRNA (NT) or ERG siRNA (E1). (A) BrdU nuclear staining of proliferating cells. In the presence of PGE2, VCaP cells with control NT siRNA showed dramatically increased BrdU nuclear staining in response to PGE2 treatment. In contrast, ERG siRNa transfected VCaP cells show significantly decreased BrdU nuclear staining both in the presence or absence of PGE2. (B) Relative percent of BrdU positive cells. In control NT siRNA transfected cells, BrdU incorporation is higher in PGE2 treated cells than in the untreated group. In contrast, BrdU incorporation is low in both ERG siRNA treated groups irrespective to PGE2 treatment.
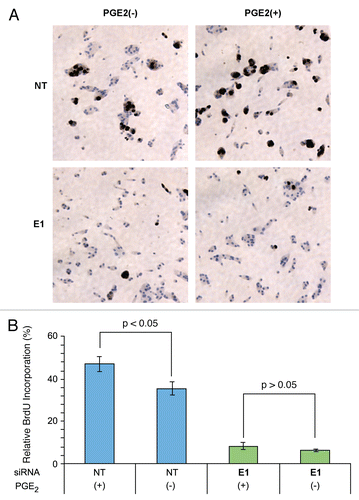
Figure 4 EP4 levels are reduced and uPA induction is inhibited in response to ERG knockdown. (A) EP4 protein levels were evaluated by immunoblot assays. Result shows that EP4 is downregulated in response to ERG knockdown. (B) expression of upa was assessed by immunoblotting at 1, 2, 4, 8 and 12 hours time points in VCaP cells treated with PGE2 as described in the Methods and Materials. PGE2 induces uPA in NT siRNA treated cells, whereas, ERG knockdown prevents uPA induction.
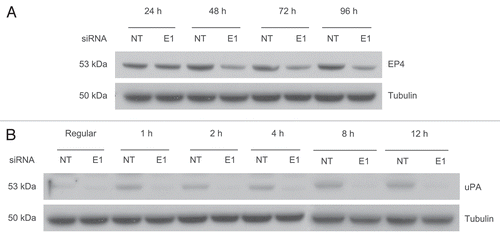
Figure 5 HPGD transcript levels are lower in TMPRSS2-ERG fusion positive prostate tumor epithelial cells than in fusion negative tumor cells. Total RNa isolated from LCM prostate epithelial tumor cells and VCaP cells were used for evaluation of HPGD and GAPDH expression. Tissue expression levels of HPGD normalized for GAPDH are shown in comparison to the normalized expression levels in VCaP cells. Evaluation of HPGD expression between TMPRSS2-ERG fusion positive (+) and negative (−) prostate tumors revealed a trend towards decreased HPGD RNA expression in fusion positive tumors.
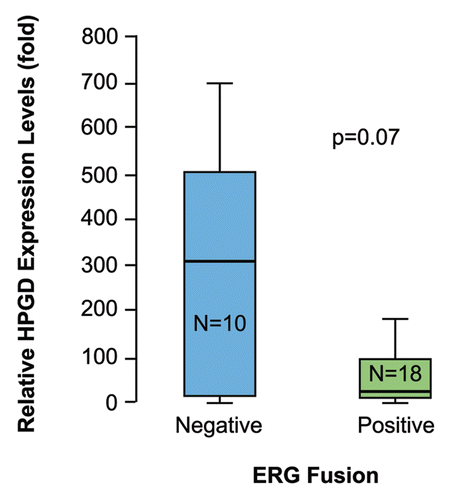
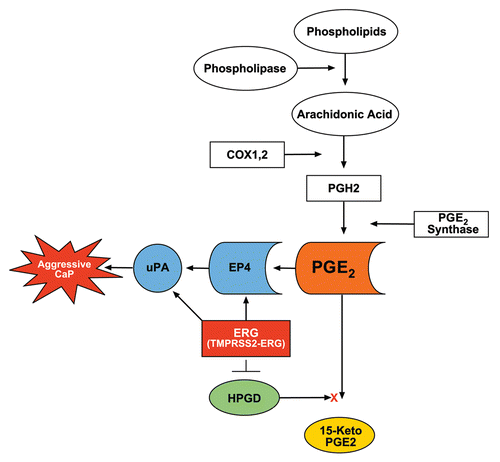
Figure 6 Proposed model for ERG functions in prostaglandin signaling pathway. Inhibition of HPGD as result of TMPRSS2-ERG overexpression prevents PGE2 catabolism, thus accumulation of PGE2 will result in uPA activation and cell growth, contributing to the progression of CaP. ERG directly binds to the promoter of uPA.