Figures & data
Figure 1 Identification of p53-interacting proteins from gradient-purified mitochondria. (A) 500 µg of mitochondrial lysate was purified from Saos2 cells containing temperature sensitive p53 (mutant conformation at 39°C and wild-type conformation at 32°C), or WM115 cells treated with camptothecin (CPT) and immunoprecipitated with polyclonal p53 antibody, run on SDS-PAGE and stained with Coomassie Blue. Arrows point to bands identified by mass spectrometry to be p53 and caspase-3; the band just above p53 is immunoglobulin heavy chain. (B) Peptide sequence of human caspase-3, with the tryptic peptides identified in (A) outlined in bold.
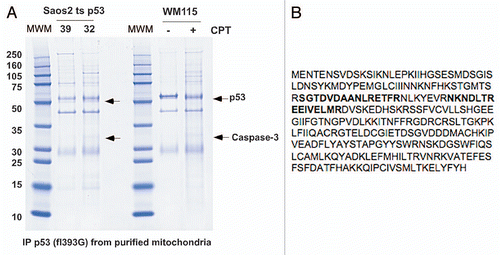
Figure 2 Caspase-3 binds endogenous p53. (A) Whole cell lysates from WM115 cells treated with and without 5 µM Camptothecin (CPT) for 6 h were immunoprecipitated (IP) with antibodies for caspase-3 (Casp3) and IgG. Western blot was performed for both p53 and caspase-3. (B) Mitochondria were purified from WM115 cells treated with and without 5 µM Camptothecin (CPT) for 6 h and immunoprecipitated (IP) with antibodies for IgG and caspase-3 (Casp 3). The blot was probed with an antibody for p53. (C) Whole cell lysates from U2OS cells that were treated with 0.5 µg/ml Adriamycin (Adr) for 24 h and immunoprecipitated (IP) with antibodies for caspase-3 (Casp 3) or Bak. Right: western analysis of the U2OS lysates used in the immunoprecipitation for p53, Caspase-3 and actin. (D) Mitochondria from H1299 Tetracycline (Tet) inducible p53 cells were isolated before and after 0.75 µg/ml Doxycycline (Doxy) treatment for 3 h. The mitochondria were immunoprecipitated (IP) with antibodies for caspase-3 (casp3), Bak and IgG. Right: western analysis was done on cytosolic (Cyto) and mitochondrial (Mito) fractions from lysates used in the immunoprecipitation. Blots were probed for GRP75 (control for mitochondria), p53 and PCNA (control for nuclear/cytosolic contamination). (E) Whole cells lysates from Saos2 cells containing temperature-sensitive forms of p53 encoding proline at amino acid 72 (P72) or arginine 72 (R72) were immunoprecipitated with antisera specific for pro-caspase-3 (E3, Santa Cruz Biotechnology) following temperature shift and wild-type p53 induction for the hours indicated. The bottom panel depicts the steady state level of p53 in whole cell lysate. (F) Immunoprecipitation of caspase-3 and p53 from H1299 cells containing tetracycline-inducible versions of the p53 polymorphic variants P72 and R72, followed by western analysis for associated p53. The right part depicts western analysis of whole cell lysates from these samples.
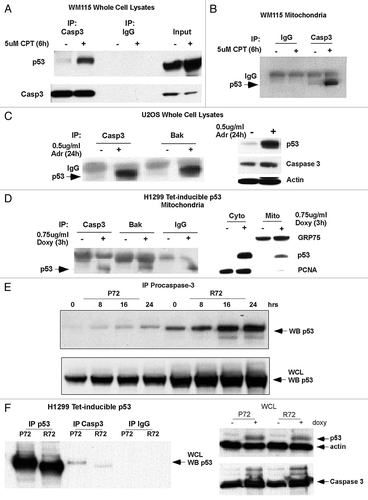
Figure 3 Caspase-3 appears to be predominantly associated with the outer mitochondrial membrane. (A) Cytosolic (Cyto) and mitochondrial (Mito) extracts from H1299 cells were analyzed by western blot for caspase-3 (Casp 3), cytochrome c (Cyto C), GRP75 (control for mitochondria), HSP 60 (control for mitochondria) and PCNA (control for nuclear/cytosolic contamination of mitochondria). (B) Mitochondria from H1299 cells were treated with increasing concentrations of trypsin for 20 min. Western analysis was performed for caspase-3 (Casp 3), Bcl-XL, cytochrome c (Cyto C) and GRP75.
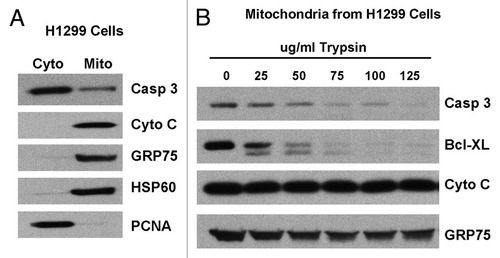
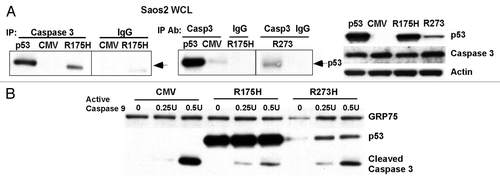
Figure 4 Mutant p53 binds to caspase-3 and inhibits the proteolytic processing of caspase-3 by active caspase-9. (A) Left: immunoprecipitates of whole cell lysate (WCL) from Saos2 cells containing temperature sensitive inducible p53 (at 32°C, wild-type conformation), CMV parental vector, the R175H mutant or the R273H mutant form of p53 with caspase-3 antibody or IgG. Western analysis was performed for p53. Right: western analysis of whole cell lysates from the cell lines indicated for the steady state level of p53, caspase-3 and actin. (B) Purified mitochondria from Saos2 cells stably expressing CMV parental vector or the R175H and R273H mutants of p53 were incubated with increasing units (U) of active caspase-9 for 30 min. Western analysis was performed using antisera specific for p53 and cleaved caspase-3.