Figures & data
Figure 1. Sodium butyrate induces H2AX phosphorylation in E1A+Ras cells. (A) Western blotting of total cell proteins probed with antibodies to Ser139-H2AX and GAPDH (loading control) at various time points of NaBut treatment. (B) Analysis of SSB DNA breaks in E1A + Ras cells. E1A + Ras cells were subjected to single-cell gel electrophoresis under denaturing conditions (comet assay): (1) untreated cells, (2) NaBut-treated cells, (3) cells irradiated at dosage 6 Gy. (C and D) Bivariate distributions (DNA content vs γH2AX) of E1A + Ras cells treated with 4 mM NaBut for time intervals as indicated. Results are present as DNA content (X-axis) vs γH2AX levels (Y-axis). Fluorescence intensity was measured by flow cytometry. Based on difference in DNA content, subpopulations of cells in G1 vs S vs G2M phases of the cycle may be distinguished and gated as shown in the Ctrl sample. The mean γH2AX IF may then be calculated for each subpopulation. Cells were treated with 4 mM NaBut for 2, 4, 8 and 24 h (C) or for 24, 48 and 72 h (D).
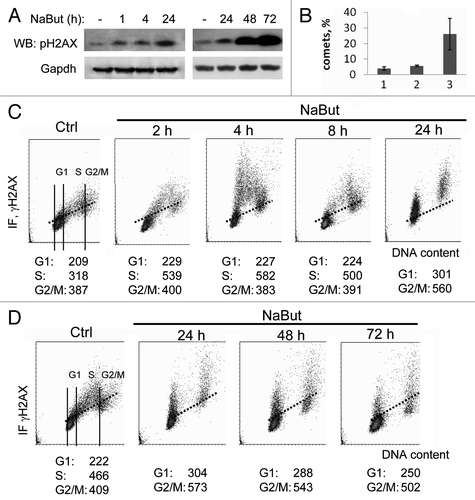
Figure 2. Induction of γH2AX foci in cells treated with adriamycin alone or in combination with NaBut. (A) Bivariate distributions (DNA content vs γH2AX) of E1A+Ras cells treated with NaBut for 24 h (NaBut) or left untreated (Ctrl) and exposed to adriamycin for 40 min (Adr) and then incubated in NaBut-free medium for 1, 4 or 18 h as indicated. (B) Immunoblotting of total proteins with antibodies to the γH2AX and GAPDH (loading control) from cells harvested 1 h or 4 h after adriamycin treatment alone or after NaBut-pretreatment for 24 h. (C) Cells were treated with adriamycin alone (light gray) or after NaBut pretreatment for 24 h (dark gray), harvested and subjected to single-cell gel electrophoresis under neutral conditions (neutral comet assay). Tail moment is defined as percentage of DNA in the tail multiplied by the distance between the means of the head and tail distributions.Citation15 Results are normalized to the tail moment in untreated cells. (D) Clonogenic survival after treatment with adriamycin alone, pretreatment with 4 mM NaBut for 24 h before adriamycin treatment, or adriamycin treatment followed by NaBut. (E) Caspase-3 activation assay. Cells were treated as indicated in previous legend. Twelve hours later, the cells were harvested, and caspase-3 activity was measured in triplicate. Results are normalized to the caspase-3 activity in untreated cells.
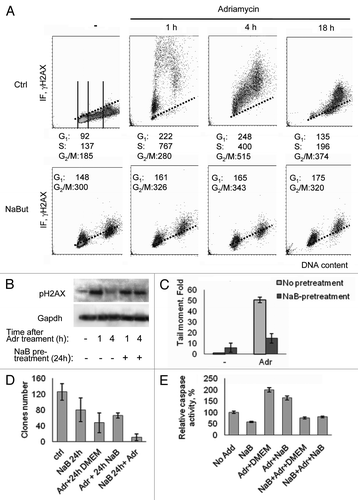
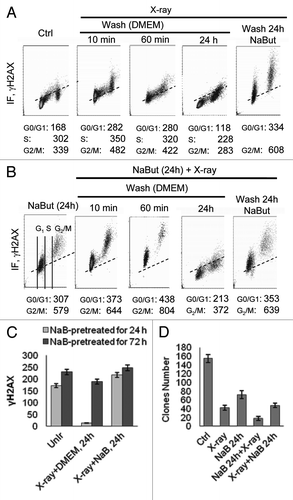
Figure 3. NaBut sensitizes E1A+Ras cells to irradiation. (A and B) Bivariate distributions (DNA content vs γH2AX) of E1A+Ras cells. (A) Cells were X-ray irradiated and incubated after irradiation for 10 min, 60 min or 24 h with or without NaBut as indicated. (B) Alternatively, cells were NaBut-pretreated for 24 h, irradiated and incubated after irradiation for 10 min, 60 min or 24 h with or without NaBut as indicated. (C) Histogram presented the dependence between duration of HDACi pretreatment and IR-induced γH2AX persistence. Phosphorylated histone H2AX was visualized by flow cytometry. To obtain the IR-induced increase in γH2AX IF (ΔH2AX IF), the means for the untreated cells were subtracted from respective means of the treated cells. Cells were pretreated with 4 mM NaBut for 24 h (light gray) or for 72 h (dark gray), irradiated and incubated after irradiation for 24 h with or without NaBut as indicated. (D) Colony formation assay after X-ray irradiation alone, pretreatment with 4 mM NaBut for 24 h before irradiation, or irradiation followed by NaBut in E1A+Ras cells. After treatment cells were trypsinized and plated as single cells in NaBut-free medium for determination of colony-forming efficiency. In each case, colonies were determined 10–14 d afterwards. Values represent the mean from three independent experiments.