Figures & data
Figure 1 Glutamine increases the mitochondrial mass of MCF7 cells cocultured with fibroblasts. (A) Fibroblast-MCF7 cell cocultures were maintained in media containing high glutamine (right) or no glutamine (left) for 5 d. Cells were then fixed and immuno-stained with antibodies directed against the intact mitochondrial membrane (red) and K8/18 (green). Nuclei were counterstained with DAPI (blue). The upper panels show the mitochondrial staining (red channel). The bottom panels show the K8/18 staining (green channel) to identify the MCF7 cell population. Note that high glutamine increases the mitochondrial mass specifically in MCF7 cells as compared with cocultures maintained without glutamine. However, glutamine does not promote mitochondrial biogenesis in the fibroblasts. Original magnification, 40x. (B) Fibroblasts and MCF7 cells were maintained as single cell-type cultures in high glutamine media for 5 d. Cells were fixed and immunostained with antibodies directed against the intact mitochondrial membrane (red) and K8/18 (green). Nuclei were counter-stained with DAPI (blue). The upper panels show the mitochondrial staining (red). The bottom panels show K8/18 staining (green) labeling MCF7 cells. Note that when cultured as single cell-types in high glutamine conditions, the mitochondrial mass is slightly higher in fibroblasts than in MCF7 cells. Original magnification, 40×.
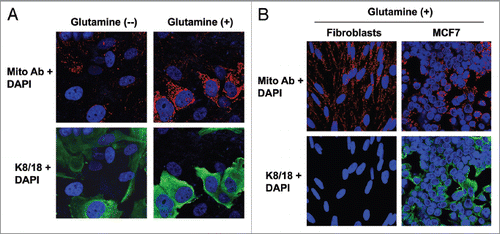
Figure 2 Glutamine induces the downregulation of Cav-1 in the fibroblast compartment. MCF7 cells and fibroblasts were cocultured for 5 d in media containing either high glutamine (right) or no glutamine (left). Cells were fixed and immunostained with antibodies against Cav-1 (red) and K8/18 (green). Nuclei were counter-stained with DAPI (blue). K8/18 staining is specific for MCF7 cells and Cav-1 is specific for fibroblasts since MCF7 cells lack Cav-1 expression. Note that Cav-1 expression is decreased in the fibroblast compartments of samples cultured with high glutamine, compared with no glutamine media. Original magnification, 40×.
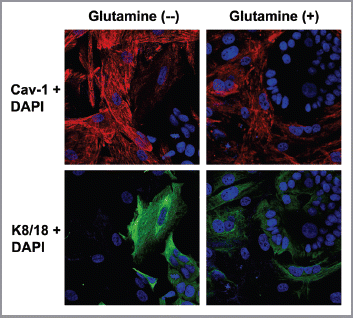
Figure 3 MCF7 cells in coculture show increased glutamine catabolism, but decreased glutamine synthesis. To evaluate glutamine metabolism, MCF7 cells were cultured alone or with fibroblasts for 5 d in high glutamine media. Then, cells were fixed and immunostained with antibodies against K8/18 (green) and GLUL (A, red) or GLS (B, red) or GLUD1 (C, red). Nuclei were counter-stained with DAPI (blue). K8/18 was used to distinguish MCF7 cells from fibroblasts. (A) Glutamine neosynthesis is decreased in MCF7 cells in coculture. GLUL catalyzes the synthesis of glutamine from glutamate and ammonia. Note that GLUL is highly expressed in single cultures of MCF7 cells. However, GLUL expression is decreased in MCF7 cells cultured with fibroblasts. (B and C) Glutamine catabolism is increased in MCF7 cells in coculture. GLS (B) and GLUD1 (C) catalyze the first and second steps respectively of the catabolism of glutamine. Note that both GLS and GLUD1 are upregulated in MCF7 cells in coculture, compared with MCF7 cells alone. Original magnification, 40× for all.
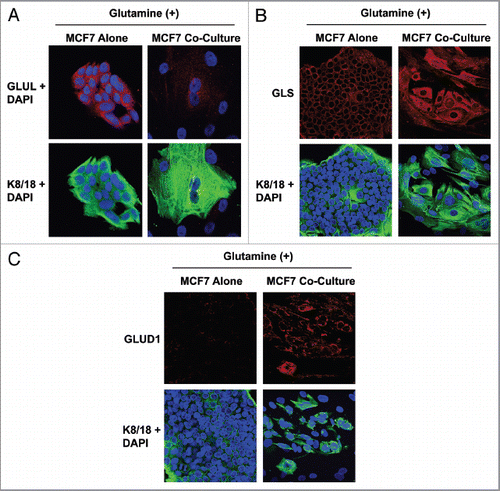
Figure 4 MCF7 cells in coculture show increased expression of the transporter for glutamine uptake, but decreased expression of the transporter for glutamine extrusion. MCF7 cells were cultured alone or with fibroblasts for 5 d in high glutamine media. Then, cells were fixed and immunostained with antibodies against K8/18 (green) and SLC6A14 (A, red) or SLC7A5 (B, red). Nuclei were counter-stained with DAPI (blue). K8/18 was used to distinguish MCF7 cells from fibroblasts. Note that SLC6A14, the transporter that facilitates glutamine uptake, is highly upregulated in MCF7 cells in coculture, compared with MCF7 cells alone. Conversely, SLC7A5, the transporter that facilitates glutamine extrusion, is greatly decreased in MCF7 cells in coculture, compared with MCF7 cells alone. Original magnification, 40×.
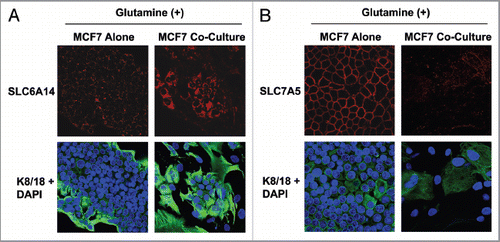
Figure 5 Chloroquine abolishes the glutamine-induced increase in mitochondrial mass in cocultured MCF7 cells, and augments apoptosis. (A) Fibroblasts were cocultured with MCF7 cells for 4 d in high glutamine media. Then, cells were treated either with 25 µM chloroquine (right) or vehicle alone (left) for 24 h. Cells were fixed and immunostained with antibodies against K8/18 (green) and the intact mitochondrial membrane (red). Nuclei were counter-stained with DAPI (blue). K8/18 specifically stains MCF7 cells (lower panels). The upper panels show the red channel only. Note that chloroquine treatment abolishes the glutamine-induced increase of mitochondrial mass of cocultured MCF7 cells. Original magnification, 40×. (B) MCF7 cells were cultured alone or in coculture with fibroblasts in the presence of chloroquine or vehicle alone (CTRL) under high glutamine conditions. Apoptosis was measured with PI staining. The percentage of apoptotic MCF7 cells (PI positive) is shown. Note that chloroquine does not induce apoptosis in MCF7 cells cultured alone, but does induce a 1.8-fold increase in apoptosis in MCF7 cells cocultured with fibroblasts.
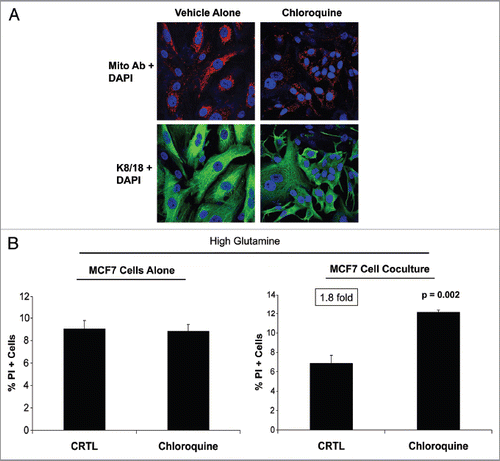
Figure 6 Glutamine decreases autophagy in MCF7 cells, but increases autophagy in fibroblasts. Single cultures of fibroblasts (A) and MCF7 cells (B) were incubated with high glutamine or no glutamine media for 3 d. Cells were then lysed and subjected to immuno-blot analysis with a panel of autophagy markers. Equal loading was assessed by β-actin immunoblotting. Note that high glutamine strongly induces the expression of autophagy markers (Lamp-1 and Cathepsin B) in the fibroblasts (A). However, high glutamine decreases the expression of autophagy markers (Beclin-1 and Lamp-1) in MCF7 cells (B). These results suggest that the glutamine-mediated effects on autophagy are compartment-specific.
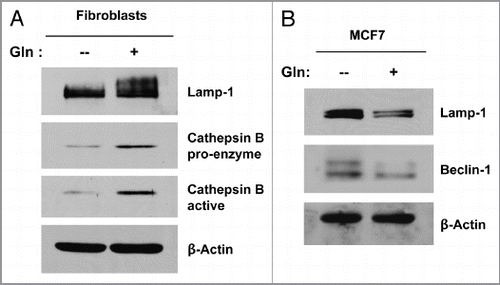
Figure 7 Glutamine protects MCF7 cells against apoptosis, with increased TIGAR expression. MCF7 cells were cultured alone in media containing high glutamine or high glucose (without glutamine) for 3 d. (A) Apoptosis was measured with annexin V and PI staining. The left graph shows the percentage of Annexin V-positive cells (early and late apoptosis). The right graph shows the percentage of total cell death (Annexin V-positive and/or PI-positive). Note that MCF7 cells cultured alone with high glutamine show an up to 2-fold decrease in apoptosis, as compared with MCF7 cells maintained in high glucose media. P values are as shown. (B) Cells were lysed and subjected to immuno-blot analysis with antibodies directed against the anti-apoptotic protein TIGAR. Equal loading was assessed by β-actin immunoblotting. Note that high glutamine increases the expression of TIGAR in MCF7 cells, compared with high glucose media.
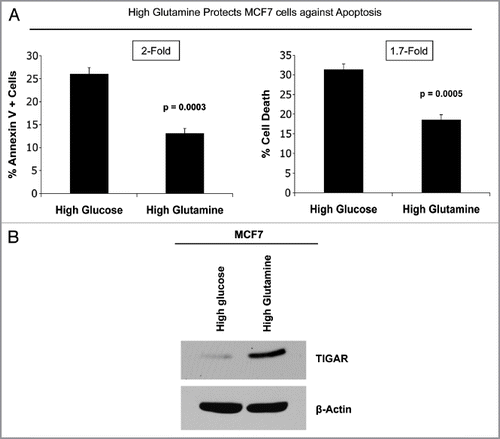
Figure 8 Glutamine protects MCF7 cells from tamoxifen-induced apoptosis. MCF7 cells either in homotypic culture or cocultured with fibroblasts were maintained either in high glutamine (Gln) or no glutamine conditions for 4 d. Then, cells were treated with tamoxifen (12 µM) or vehicle alone (CTR) for 24 h. Apoptosis was measured with annexin V and PI staining. The percentage of apoptotic or dead MCF7 cells (Annexin V positive and/or PI positive) is shown. (A and B) For simplicity of explanation, the same graph is shown twice in (A and B). (A) MCF7 cells alone. MCF7 cells alone cultured with high glutamine show a 3.3-fold decrease in apoptosis compared with cells cultured with no glutamine (first and second bars). Interestingly, high glutamine protects MCF7 cells from tamoxifen-induced apoptosis. Note that MCF7 cell tamoxifen-induced apoptosis is decreased by 1.5-fold in high glutamine conditions, as compared with no glutamine (third and fourth bars). Cocultured MCF7 cells. Note that under untreated conditions, cocultured MCF7 cells with high glutamine show a 1.9-fold decrease in apoptosis compared with cells cultured with no glutamine (fifth and sixth bars). However, high glutamine greatly protects cocultured MCF7 cells against tamoxifen-induced apoptosis. Note that upon tamoxifen treatment, cocultured MCF7 cells in high glutamine show a 3.4-fold decrease in apoptosis, compared with cells cultured with no glutamine (seventh and eighth bars). These results indicate that glutamine protects MCF7 cells fromtamoxifen-induced apoptosis, and that glutamine and fibroblasts cooperate to confer tamoxifen-resistance. P values are as shown. (B) Fibroblasts cooperate with glutamine to confer tamoxifen-resistance. In untreated control conditions (CTR), cocultured MCF7 cells maintained with glutamine show a 1.5-fold reduction in apoptosis compared with MCF7 cells alone (second and sixth bars). However, upon tamoxifen treatment in the presence of glutamine, fibroblasts induce a 4.3-fold decrease in MCF7 cell apoptosis (fourth and eighth bars). P values are as shown.
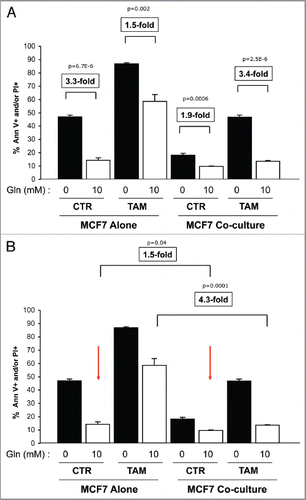
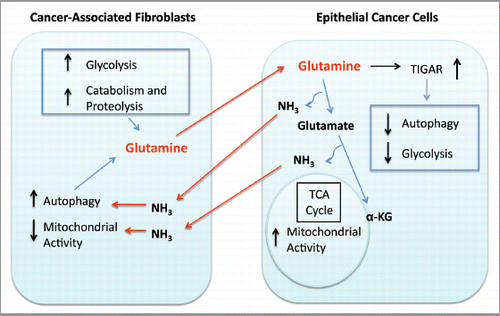
Figure 9 Glutamine addiction and stromal autophagy: a vicious cycle. Our current data suggest that metabolic-coupling occurs between epithelial cancer cells and stromal cells. Cancer-associated fibroblasts undergo an autophagic program, leading to the generation and secretion of high glutamine levels into the tumor microenvironment. Cancer cells accumulate glutamine and convert it to glutamate and ammonia. Glutamate is further catabolized to α-ketoglutarate, which enters the TCA cycle and increases the mitochondrial activity of epithelial cancer cells. Epithelial cancer cells are protected from apoptosis by the upregulation of TIGAR, an endogenous inhibitor of glycolysis, apoptosis and autophagy. The other glutamine byproduct, ammonia, freely diffuses into the microenvironment, and induces autophagy and glutamine production in CAFs. Finally, the use of autophagy inhibitors such as chloroquine can uncouple the epithelial and stromal compartments, resulting in decreased mitochondrial activity in epithelial cancer cells ( and ).