Figures & data
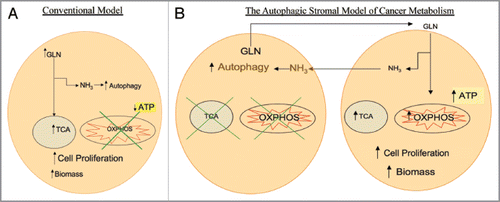
Figure 1 (A) Conventional model of cancer metabolism. Cancer cells utilize glutamine (GLN) to replenish tricarboxylic acid (TCA) cycle intermediates, a process termed anaplerosis, and to increase cellular biomass. The catabolism of glutamine generates ammonia (NH3), inducing autophagy. It has been postulated that cancer cells have impaired oxidative phosphorylation (OXPHOS) with low ATP generation with high autophagy which promotes cell proliferation. (B) The autophagic stromal model of cancer metabolism as proposed by the authors. Cancer associated stromal cells possess high levels of autophagy and impaired mitochondrial activity, leading to release of catabolites including glutamine which are taken up by cancer cells. Glutamine in the cancer cells not only leads to high ammonia generation that drives autophagy in stromal cells, but also increases the mitochondrial TCA cycle and oxidative phosphorylation, leading to high levels of ATP production, increased biomass and proliferation of cancer cells. This reciprocal feed-forward communication supports both tumor progression and continued activation of the surrounding stroma, compromising cancer therapy.