Figures & data
Figure 1 Relative expression of CD20 on lymphoma B-cell lines. B-cell lines were tested for CD20 expression and analyzed by flow cytometry. Histograms of CD20 fluorescence intensity are shown compared with the IgG1 isotype. The results presented are representative of at least three independent experiments.
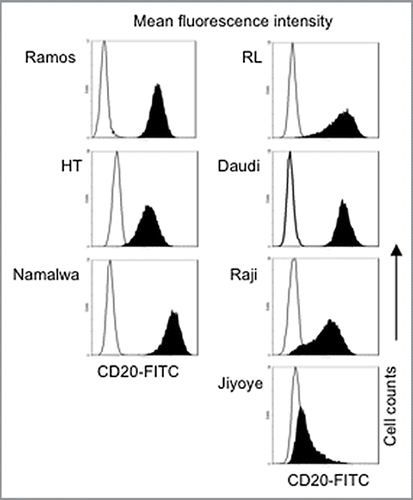
Figure 2 Combination of plitidepsin and rituximab synergistically inhibit B cells. Dose-response curves (log scale) of cells cultured in RPMI plus 10% non-heat inactivated FBS exposed to plitidepsin, rituximab and their combination (A), compared with doxorubicin, rituximab and their combination (B), after 96 h of treatment. (C) Sequence of drug concentrations at a fixed plitidepsin:rituximab dose ratio with the IC50 at 1.25 nM: 2.17 nM.
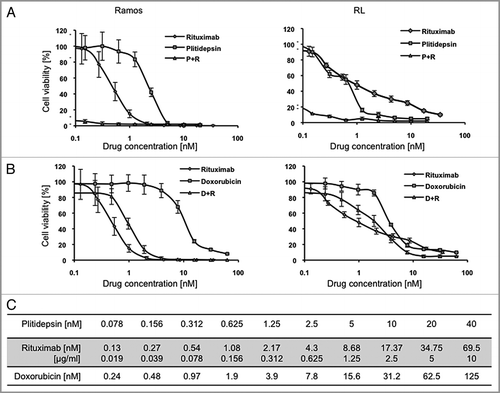
Figure 3 Plitidepsin blocks cells in the G1/S phase of the cell cycle. Ramos (A), and RL cells (B), were incubated with rituximab, plitidepsin, or their combination at 37°C for 24 h. Cells (1 á 105) were stained with 0.5 ml PI/RNase staining buffer and analyzed by flow cytometry with CellQuest software (Becton-Dickinson). Results analyzed with the Modfit software are presented as the mean and SD from three independent experiments. In Ramos cells p < 0.005 only for the plitidepsin treatments in comparison with the control. In RL cells p < 0.005 for all treatments in comparison with the control. (C) Histograms of the cell cycle fluorescence intensity after 24 h treatment with each drug alone and in combination.
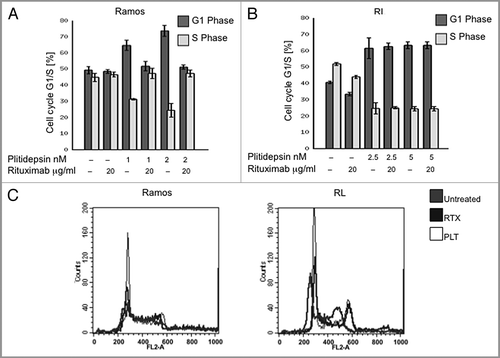
Figure 4 Apoptosis induced by plitidepsin, rituximab or their combination in rituximab-sensitive B cells. Ramos and RL cells were incubated with rituximab, plitidepsin, or their combination at 37°C for 48 h. Cells (1 × 105) were stained with Annexin V-FITC and 7AAD and analyzed by flow cytometry. Data are representative of three independent experiments. (A) Y axis represents the apoptotic cells. In Ramos and RL cells p < 0.001 for the rituximab treatments alone and in combination with plitidepsin in comparison with the control. (B) In the histograms, FL-1 (Annexin V-FITC) thresholds were determined by using cells stained without Annexin buffer per manufacturer's protocol. (C) Expression of caspase-3 and caspase-7 was analyzed by western blotting in Ramos cells treated with plitidepsin (C), plitidepsin plus rituximab (D) and rituximab (E) for the indicated times.
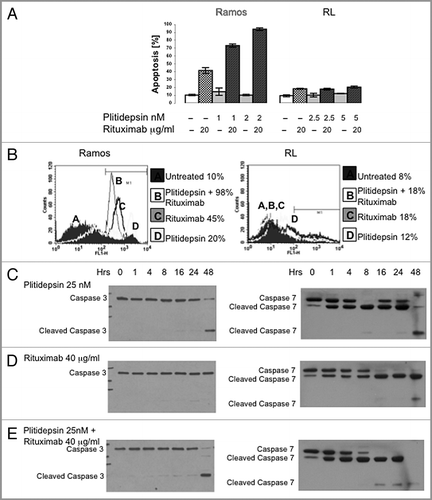
Figure 5 Western blot analysis of caspase activation in B cells expressing different levels of CD20. B cells with different levels of resistance to rituximab were treated for the indicated times with plitidepsin, plitidepsin plus rituximab and rituximab. Expression of caspase-7 (A) and caspase-3 (B) was analyzed by western blotting.
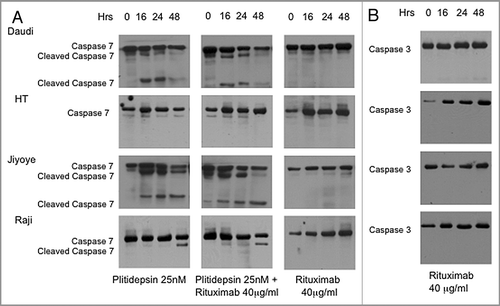
Figure 6 The effect of plitidepsin, rituximab and the combination of these drugs in Ramos tumor xenografts in mice. (A) Ramos cells (107) were inoculated subcutaneously in the flanks of irradiated athymic nude female Ncr mice. When the tumor diameter reached 0.5 cm, mice were simultaneously treated with rituximab (200 µg/kg), plitidepsin (0.2 or 0.4 mg/kg), or the combination of these drugs at four doses 3 d apart beginning on day 15 after tumor cell inoculation. Tumor measurements were taken at 2 d intervals (B) Survival of the mice treated with plitidepsin or rituximab alone, and in combination. Survival curves between control and each drug and control and combination treatment was significant (p = 0.03).
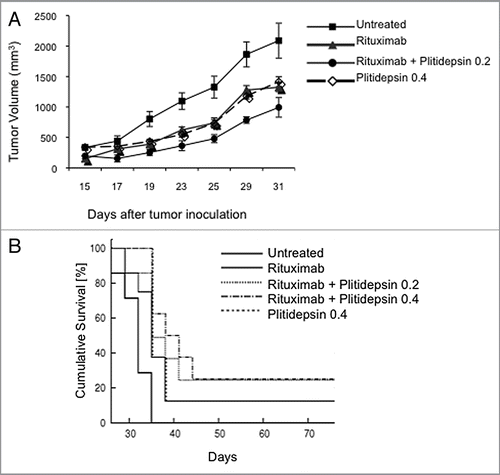
Table 1 Effect of rituximab and plitidepsin on lymphoma B-cell lines