Figures & data
Table 1. AZD4877-induced apoptosis in human bladder cancer cells
Figure 1. Effect of AZD4877 treatment on a panel of bladder cancer cell lines. Propidium iodide staining and FACS analysis were used to determine effects of AZD4877 (10nM) on apoptosis at 24 (A) and 48 (B) h time points. Percentages of subG0/G1 cells in treated and untreated cells is shown on the Y-axis (n = 3). In (C), the effects of AZD4877 (10 nM, 24 h) and cisplatin (10 μM, 48 h) were compared by subtracting background DNA fragmentation levels from the levels observed in drug-exposed cells.
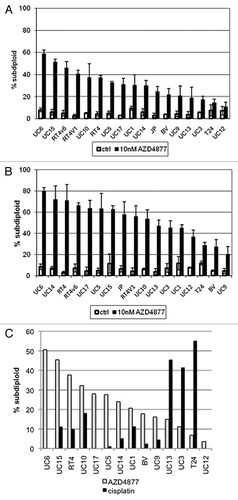
Table 2. Differences in baseline gene expression in AZD4877-sensitive and -resistant bladder cancer cells
Figure 2. Differential p63 expression in AZD4877-sensitive and -resistant bladder cancer cell lines. (A) Expression of p63, E-cadherin (CDH1), and P-cadherin (CDH3) were measured using Illumina whole genome mRNA expression profiling. p63 levels were confirmed in a 10 cell line subset by immunoblotting (B) and quantitative RT-PCR (C).
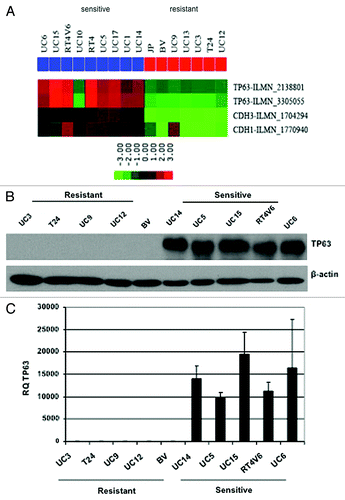
Figure 3. Effect of p63 knockdown on drug sensitivity in UC14 cells. (A) Parental UC14 cells, as well as cells transfected with non-targeting and p63 shRNA constructs were exposed to 10 nM AZD4877 for 24 h and apoptosis was measured by propidium iodide staining and FACS analysis. (B) Cells were transfected with non-targeting or ΔNp63-specific siRNAs, and the effects of AZD4877 on PARP cleavage were measured by immunoblotting. Blots were then stripped and reprobed with anti-p63 (4A4) to confirm p63 knockdown and subsequently with anti-tubulin to confirm equal loading. (C) Parental UC14 cells, as well as cells transfected with non-targeting and p63 shRNA constructs were exposed to10 ng/ml docetaxel for 24 h and apoptosis was measured by propidium iodide staining and FACS analysis (n = 3). (D) Cells were exposed to 1 μM gemcitabine or 10 μM cisplatin as single agents or in combination for 48 h, and apoptosis was measured by propidium iodide staining and FACS analysis (n = 3).
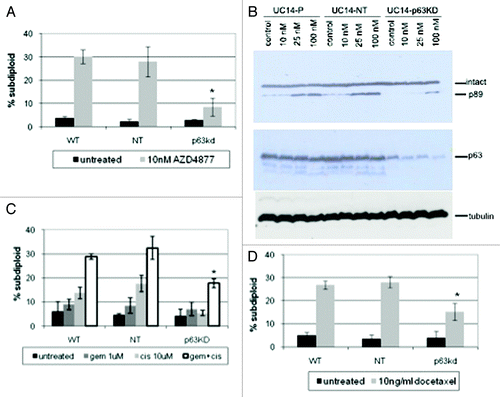
Figure 4. Effect of p63 knockdown on proliferation and c-myc expression. Parental UC14 cells, as well as cells stably transduced with non- targeting and p63 specific shRNAs, were grown in 6-well plates and quantified by trypan blue exclusion at 24, 48 and 72 h time points (A; n = 3). Real-time RT-PCR (B) and western blotting (C) were then used to confirm p63 silencing and to measure Myc expression in the 3 UC14 variants. Myc expression was quantified by densitometry as a ratio to actin expression using ImageJ.
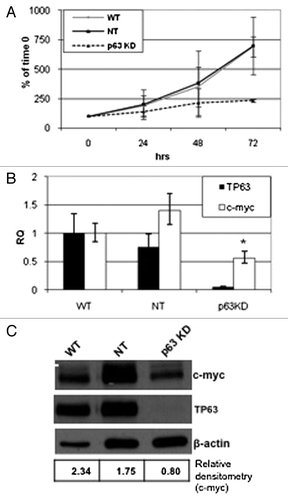
Figure 5. Relationship between β-catenin and p63 expression. Western blotting was used to measure β-catenin and Myc expression levels in a panel of 10 human bladder cancer cell lines (A), and in UC14 cells transfected with non-targeting shRNA and shRNA specific for p63 (B). β-catenin levels were quantified as a ratio to actin expression using ImageJ.
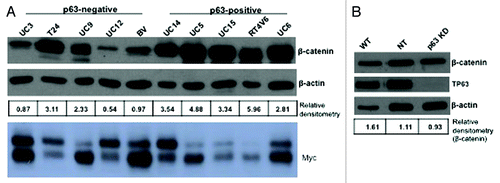
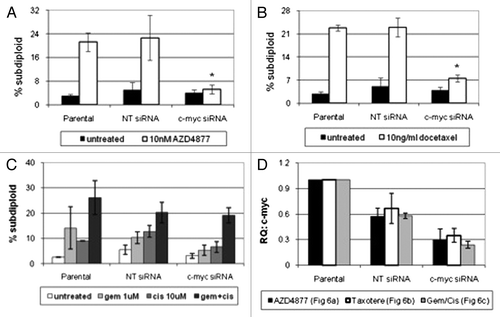
Figure 6. Effect of c-myc silencing on sensitivity to AZD4877 or docetaxel in UC14 cells. Parental UC14 cells, as well as cells transfected with non-targeting or c-myc- specific siRNAs, were exposed to 10 nM AZD4877 for 24 h (A) or to 10 ng/ml docetaxel (B) for 24 h. Additionally, cells were exposed to gemcitabine (1 μM), cisplatin (10 μM) or a combination of the two drugs for 48 h (C). Propidium iodide staining and FACS analysis were used to measure apoptosis. Confirmation of c-myc silencing for all experiments is shown in (D) (n = 3).