Figures & data
Table 1. Clinicopathological features and anti-TRPM8 immunoreactivity of pancreatic adenocarcinoma in a mini-series of five patients
Figure 1. Radiographic and pathological features of pancreatic adenocarcinoma with metastasis to regional lymph nodes. (A) computed tomograph showing heterogeneous attenuation in the head and neck region of the pancreas (green arrow) with abrupt termination of common bile duct and pancreatic duct consistent with pancreatic cancer. (B) endoscopic ultrasonograph showing a large, hypoechoic mass (indicated diagonally by yellow and blue dotted lines) in the head of the pancreas with irregular outer margins. (C–F) histology of resected pancreatic adenocarcinoma stained with hematoxylin and eosin. (C) pancreatic tumor that contains atypical glands infiltrating a markedly desmoplastic stroma consistent with well-differentiated adenocarcinoma (20x magnification). (D) pancreatic adeno-carcinoma, in which atypical glandular epithelia contain large, pleomorphic nuclei with prominent nucleoli (200x magnification). (E and F) peri-pancreatic lymph nodes containing metastatic adenocarcinoma (20x magnification).
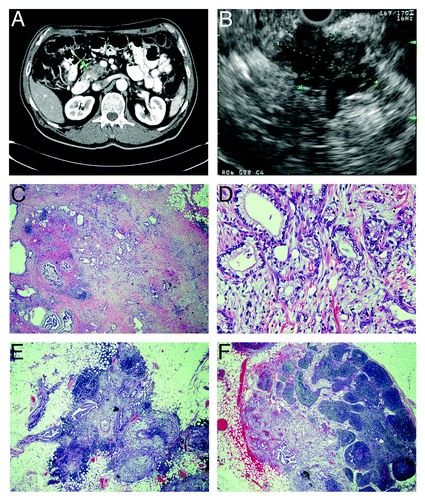
Figure 2. TRPM8 is aberrantly expressed in pancreatic adenocarcinoma. The paraffin-embedded tumor sections from the surgically resected specimen were analyzed by immunohistochemistry using rabbit anti-human TRPM8 antibodies (Lifespan Biosciences) at 1:50 dilution, followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (EnVision+ System, Dako). The signals were detected by color reaction using 3,3′-diaminobenzidine (Dako), counterstained with hematoxylin (Richard-Allan Scientific), and mounted using Permount (Sigma). The brown color indicates the presence of TRPM8 protein. Strong immunoreactivity against TRPM8 can be observed in the ductal epithelia of the pancreatic adenocarcinoma, with staining in both plasma membrane and cytoplasm. There is heterogeneity for expression of TRPM8 in the tumor. (A) 40x magnification. (B) 200x magnification. (C) 400x magnification. Tissue sections incubated in the absence of anti-TRPM8 antibodies were processed in parallel as control, and they did not exhibit any detectable immunoreactivity against TRPM8 (data not shown).
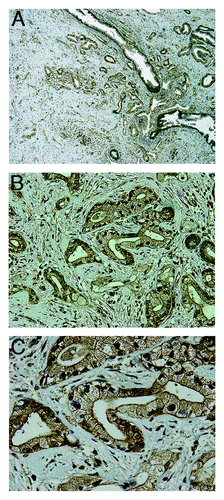
Figure 3. Expression of TRPM8 in pancreatic adenocarcinoma tissues. Surgically resected specimens were embedded in paraffin and processed for immunohistochemistry using rabbit anti-human TRPM8 antibodies (Lifespan Biosciences) followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (EnVision+ System, Dako). The signals were detected by color reaction using 3,3′-diaminobenzidine (Dako), counterstained with hematoxylin (Richard-Allan Scientific), and mounted using Permount (Sigma). The brown color indicates the presence of TRPM8 protein. Note the heterogeneity for expression of TRPM8 in the tumors. Controls using the consecutive tissue sections for each paraffin block were processed in the absence of anti-TRPM8 antibodies, and no immunoreactivity against TRPM8 was detected (data not shown). The panels A though D correspond to the patients as described in .
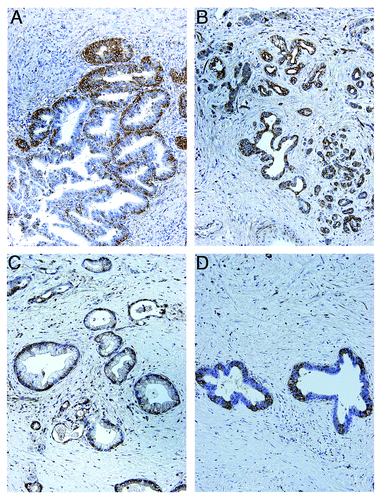
Figure 4. Anti-TRPM8 siRNA did not induce apoptotic cell death. The BxPC-3 and PANC-1 cells were transfected with anti-TRPM8 siRNA or non-targeting control siRNA by using Nucleofector® (Amaxa/Lonza) as described.Citation10 Following transfection, the cells were seeded at 2 x 105 cells / 3 ml in each well of a 6-well cell culture cluster (Corning Inc., costar) and incubated at 37°C for 72 h. The cells were then washed with phosphate buffered saline (PBS, pH 7.4), incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin V (Invitrogen) and propidium iodide (PI, Invitrogen), and analyzed for apoptosis by flow cytometry as described.Citation4 The proportion of cells undergoing early apoptosis (lower right quadrant) and late apoptosis (upper right quadrant) is indicated. The baseline level of apoptotic cells in the control siRNA-transfected group may be attributed to electrical pulsation during Nucleofection®, detachment of cells by trypsinization, and disaggregation of cells by filtration prior to flow cytometric analysis.
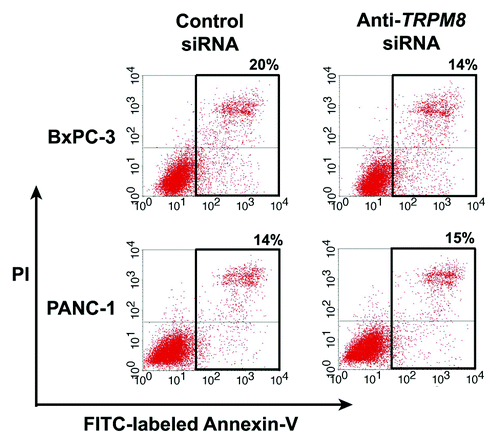
Figure 5. RNA interference-mediated silencing of TRPM8 induced replicative senescence. The BxPC-3 and PANC-1 cells were transfected with either anti-TRPM8 siRNA or non-targeting control siRNA and then seeded at 4 x 104 cells / 2 ml medium in each well of a 12-well cell culture cluster (Corning Inc., costar). The transfected cells were incubated at 37°C for 72 h, and then analyzed for activity of SA β-gal using the Senescence Detection Kit (Biovision Medical Products) according to the manufacturer’s instructions. Images were acquired under an inverted light microscope with phase contrast (Nikon TE-300) and processed using Adobe Photoshop CS3 extended. Appearance of blue color indicates SA β-gal activity (arrows), which is detected in the anti-TRPM8 siRNA-transfected cells but not in those transfected with control siRNA.
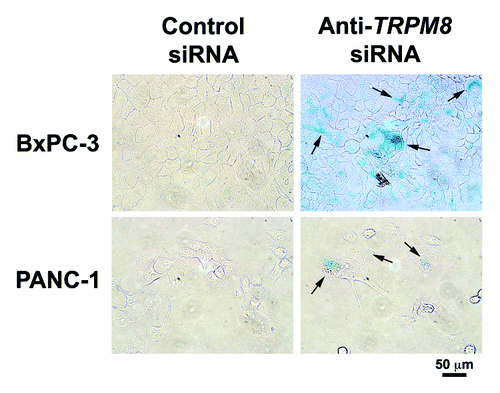
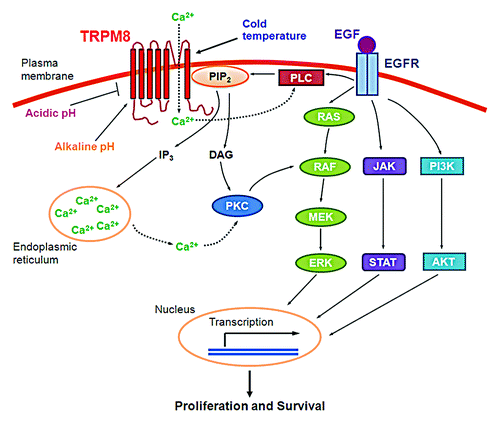
Figure 6. A working model for the proliferative and pro-survival roles of TRPM8 in pancreatic adenocarcinoma cells. Cold temperature, menthol, alkaline pH, or phosphatidylinositol-4,5-bis-phosphate (PtdIns(4,5)P2) activates the ion channel of TRPM8. Conversely, acidic pH inhibits the channel activity of TRPM8. Once activated, TRPM8 allows inflow of Ca2+ from the extracellular medium into the cytosol, leading to activation of Ca2+-sensitive phospholipase C (PLC) and hydrolysis of PtdIns(4,5)P2, thus providing negative feedback inhibition of the TRPM8 channel activity. Hydrolysis of PtdIns(4,5)P2 produces inositol 1,4,5-triphosphate (Ins(1,4,5)P3), which causes release of Ca2+ from intracellular stores and generates diacylglycerol (DAG). The increased Ca2+ or DAG activates protein kinase C (PKC). PKC in turn activates RAF in the RAS/ERK pathway,Citation34 leading to transcription of a variety of genes and resulting in cellular proliferation and survival.Citation35