Figures & data
Table 1. Pharmacokinetic Parameters of BIIB036 Administered Intraperitoneally (6.4 mg/kg) in Adult Female (Nude) Tumor Bearing Mice
Figure 1. BIIB036 inhibits tumor growth in xenograft models during and beyond the period of dosing. (A) Efficacy of BIIB036 treatment in WiDr colon carcinoma xenograft model is shown. Tumor volume as a function of time is plotted. Nude mice implanted with WiDr tumor cells were treated with varying doses of BIIB036 (25.6, 12.8, 6.4 or 3.2 mg/kg) or human Ig control (25.6 mg/kg) QW for 6 weeks. Data are mean ± SEM of n = 10 mice per group. Significant efficacy is evident at all doses relative to control group (p < 0.002 on day 83 for all dose groups). Extended efficacy > 50 d beyond the dosing period is observed. (B) Efficacy of BIIB036 treatment in MDA-MB-231 breast carcinoma xenograft model is shown. SCID mice implanted with MDA-MB-231 tumor cells were treated with varying doses of BIIB036 (25.6, 12.8 or 6.4 mg/kg) or human Ig control (25.6 mg/kg) QW for 6 weeks. Data are mean ± SEM of n = 9 mice per group. Significant efficacy is evident at all doses relative to control group (p < 0.002 on day 78 for all dose groups). Extended efficacy up to 40 d beyond the dosing period is observed. (C) Efficacy of BIIB036 treatment in NCI-N87 gastric carcinoma xenograft model is shown. SCID mice implanted with NCI-N87 tumor cells were treated with varying doses of BIIB036 (12.8, 6.4 or 3.2 mg/kg) or human Ig control (12.8 mg/kg) QW for 6 weeks. Data are mean ± SEM of n = 10 mice per group. Significant efficacy is evident at all doses relative to control group (p < 0.002 on day 78 for all dose groups). Extended efficacy > 50 d beyond the dosing period is observed.
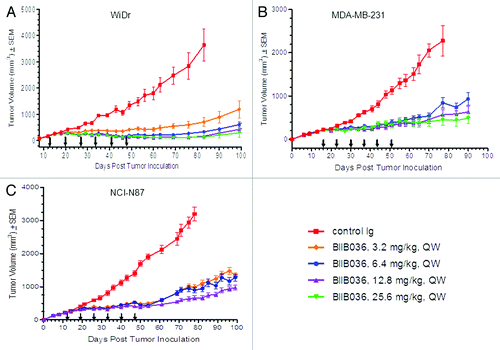
Figure 2. BIIB036 inhibits growth of large tumors upon second cycle of treatment. NCI-N87 tumor cells were implanted into CB17 SCID mice. When tumors reached ~500 mm3, one group of mice (n = 10) received a first cycle of treatment with BIIB036 (12.8 mg/kg) and one group received control Ig, administered IP QW for 6 weeks. Tumors in the BIIB036 treated group were then allowed to regrow until they reached an average size of ~975 mm3 (day 52). In a second cycle of treatment, half of the BIIB036-treated group (n = 5) was re-treated with BIIB036 (12.8 mg/kg) whereas the other half of the BIIB036-treated group (n = 5) received vehicle control, QW for 4 weeks. Data are mean ± SEM. Significant efficacy is observed in the BIIB036 treated mice following a second cycle of treatment (p < 0.0006 at day 133).
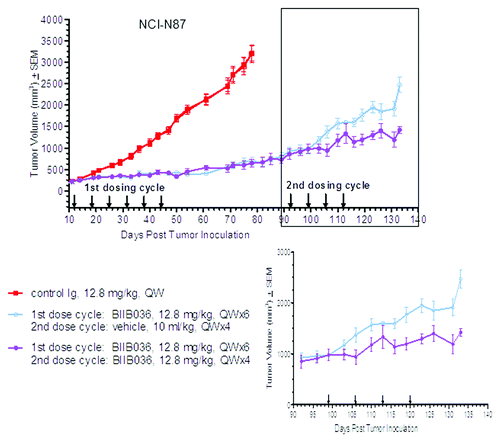
Figure 3. Maximal efficacy of BIIB036 is achieved with a Q2W dosing schedule. (A) Efficacy of BIIB036 (6.4 mg/kg, IP) administered QW, Q2W or Q3W in WiDr colon tumor xenografts is compared. Statistically equivalent efficacy throughout the study is maintained with dosing QW or Q2W, whereas tumors in the Q3W treatment group grew back more rapidly following termination of dosing. BIIB036 administered on a QW vs. Q2W is compared in the (B) MDA-MB-231 and (C) NCI-N87 tumor models, and no statistical difference in efficacy is observed. Data are mean ± SEM of n = 10 mice per group.
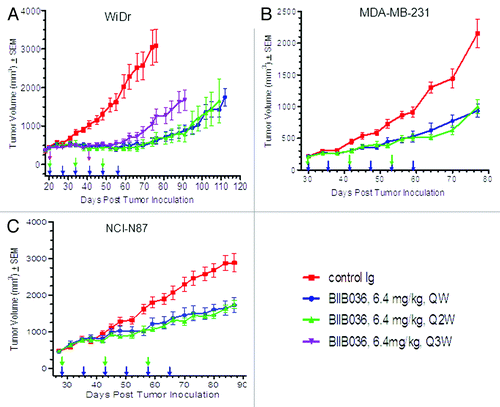
Figure 4. Equivalent efficacy of BIIB036 is observed with administration IP vs. SC Mice bearing. (A) NCI-N87 or (B) MDA-MB-231 xenograft tumors were administered BIIB036 either IP or SC on a Q2W schedule at various doses as indicated. Data are mean ± SEM of n = 10 mice per group. No statistical difference between IP and SC treatment groups at any given dose is observed.
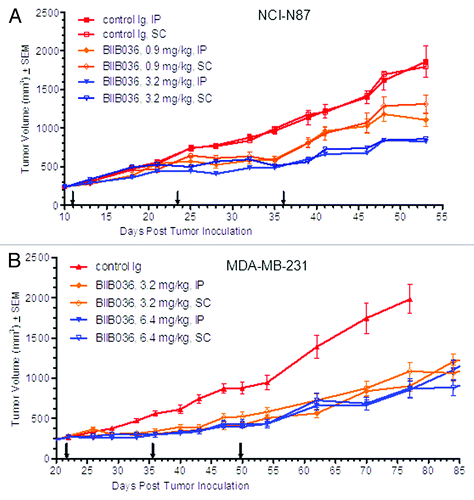
Figure 5. BIIB036 inhibits growth of patient derived primary tumors. Low passage colorectal tumor fragments derived from patients and passaged in mice were implanted into nude mice and administered treatment IP on a QW schedule. In (A) and (B), the CCR003H tumor model was tested with treatment of BIIB036 (n = 5) or control Ig (n = 10) at 6.4 mg/kg. In (C) and (D), the ST042 tumor model was tested with treatment of BIIB036 (n = 5) or control Ig (n = 10) at 3.2 mg/kg or irinotecan (n = 5; 100 mg/kg, QW). In (A) and (C), tumor growth is shown as mean ± SEM. In (B) and (D), time to progression (i.e., tumor reaches1000 mm3) is plotted as % survival.
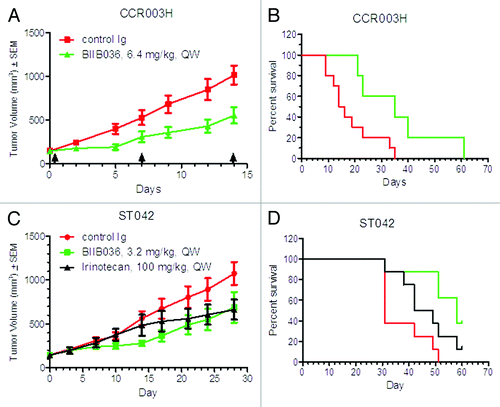
Figure 6. BIIB036 enhances efficacy of paclitaxel without overlapping toxicities. (A) MDA-MB-231 tumor bearing mice were administered BIIB036 (3.2 or 6.4 mg/kg), paclitaxel (25 mg/kg Q4X3), or a combination of BIIB036 (3.2 or 6.4 mg/kg) plus paclitaxel. Data are mean ± SEM of n = 10 mice per group. Statistically superior tumor inhibition is observed in the combination treatment as compared with single agent groups (repeated measure ANOVA, p < 0.01 for BIIB036 (6.4 mg/kg) combination relative to BIIB036 (6.4 mg/kg) or paclitaxel alone; p < 0.001 for BIIB036 (3.2 mg/kg) combination relative to BIIB036 (3.2 mg/kg) or paclitaxel alone). (B) Percent change in body weight of each group of mice from the MDA-MB-231 xenograft experiment in (A) is plotted as a function of time. No reduction in body weight is observed in any of the treatment groups.
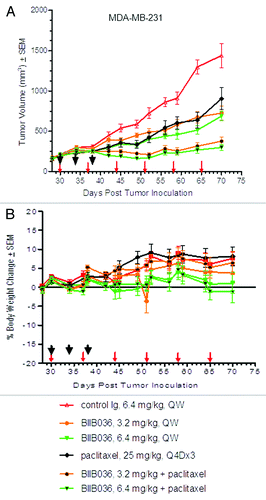
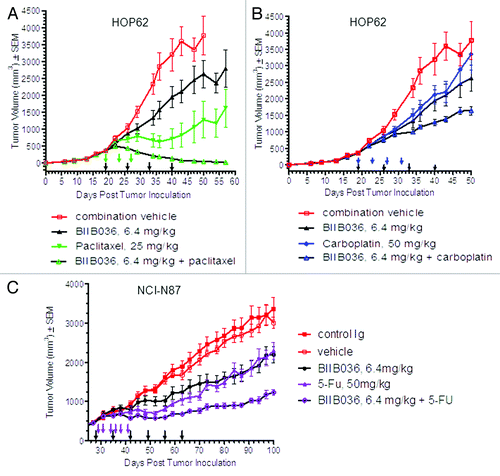
Figure 7. BIIB036 enhances efficacy of several standard of care chemotherapeutics. (A) HOP62 lung tumor bearing mice were administered paclitaxel (25 mg/kg Q4DX3), BIIB036 (6.4 mg/kg) or a combination of paclitaxel plus BIIB036 (6.4 mg/kg). Data are mean ± SEM of n = 10 mice per group. Statistically superior tumor inhibition is observed in the BIIB036 combination treatment as compared with single agent groups (repeated measure ANOVA, p < 0.0001). (B) HOP62 lung tumor bearing mice were administered carboplatin (50 mg/kg, Q4DX4), BIIB036 (6.4 mg/kg) or a combination of carboplatin plus BIIB036 (6.4 mg/kg). Data are mean ± SEM of n = 10 mice per group. Statistically superior tumor inhibition is observed in the BIIB036 combination treatment as compared with single agent groups (repeated measure ANOVA p < 0.05). (C) NCI-N87 tumor bearing mice were administered 5-FU (50 mg/kg, Q2DX6), BIIB036 (6.4 mg/kg) or a combination of 5-FU plus BIIB036 (6.4 mg/kg). Data are mean ± SEM of n = 9 mice per group. Statistically superior tumor inhibition is observed in the BIIB036 combination treatment as compared with single agent groups (repeated measure ANOVA, p < 0.001).