Figures & data
Figure 1. The stable expression of exogenous PDCD5 in U87 cells and its effect on cell morphology and proliferation. The expression of PDCD5 in U87 cells was detected by RT-PCR (A), western blot (B) and immunocytochemistry (C) after stably transfected with pcDNA3.1(MOCK) or pcDNA3.1-PDCD5 (PDCD5). PDCD5 expression was significantly increased in PDCD5-transfected cells compared with corresponding control cells. (D) The morphology of U87 cells stably transfected with MOCK (U87-MOCK) or PDCD5 plasmid (U87-PDCD5). (E) The effect of exogenous expression of PDCD5 on the growth of U87 cells.
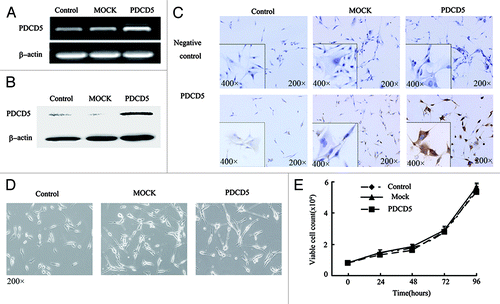
Figure 2. The effect of PDCD5 expression on the chemosensitivity of glioma cells. (A) The morphology changes of U87 cells transfected with MOCK or PDCD5 plasmid treated with different concentrations of CDDP for 48h. U87-PDCD5 cells became round after treatment with 12.5 μg/ml CDDP, while U87-MOCK cells change under the action of 25 μg/ml CDDP. (B–E) The survival of U87-MOCK and U87-PDCD5 cells was examined by MTT assay when they were exposed to CDDP, CBDCA, VCR, and VP-16 for 48 h. Values are the mean ± SEM, all the experiments were repeated three times. Overexpression of PDCD5 increased sensitivity of U87 cells to CDDP, CBDCA and VCR but had no effect on VP-16 (*p < 0.05, **p < 0.01).
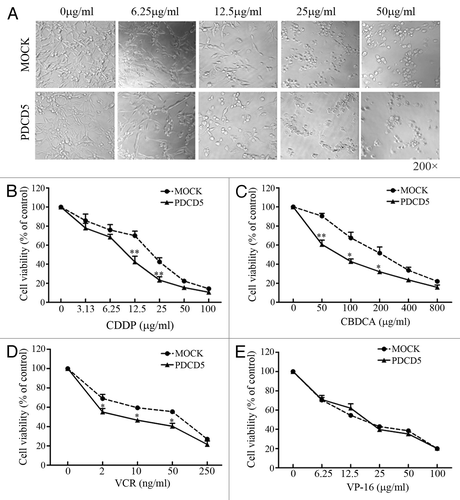
Table 1. The effect of PDCD5 overexpression on IC50 values of chemotherapeutic drugs
Figure 3. The effect of exogenous expression of PDCD5 on the growth of glioma cells treated with low doses of chemotherapeutic agents. (A) The morphology of U87-MOCK and U87-PDCD5 cells when treated with low concentration of CDDP (3 μg/ml) for 1, 4 and 9 d. (B) The cell viability of U87-MOCK and U87-PDCD5 cells when treated with low concentration of CDDP. One week later, number of viable cells in U87-PDCD5 group markedly decreased compared with that in U87-MOCK group (**p < 0.01). (C) The colony formation of U87-MOCK and U87-PDCD5 cells when treated with low concentration of CDDP (3 μg/ml) for 2 weeks. The colony-forming capacity of U87-PDCD5 cells was significantly reduced compared with U87-MOCK cells. Data are one representation of three separate experiments.
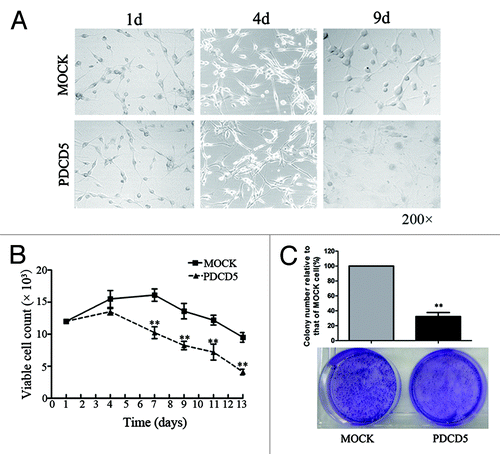
Figure 4. The effect of PDCD5 knockdown on the chemosensitivity of glioma cells to CDDP. U87-PDCD5(A), U251(B) and T98G (C) cells were transfected with 5 nM siRNA-1 (P-1), siRNA-2 (P-2) and negative control siRNA (non-specific oligonucleotides, NC), 48 h after transfection, the expression of PDCD5 were detected by RT-PCR and western blot. PDCD5 expression was significantly decreased in cells transfected with siRNA-2 (P-2). The chemosensitivity of U87-PDCD5 (D), U251 (E) and T98G (F) cells to CDDP after knockdown of PDCD5 by siRNA. Knockdown of PDCD5 significantly decreased sensitivity of U87-PDCD5 cells and U251 cells to CDDP, and slightly in T98G cells. Data are expressed as the mean of triplicates ± SEM (*p < 0.05, **p < 0.01).
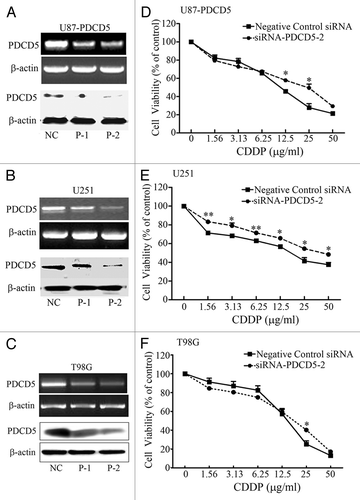
Table 2. The effect of PDCD5 silence in three glioma cell lines on IC50 values of CDDP
Figure 5. The effect of exogenous expression of PDCD5 on CDDP-induced apoptosis in glioma cells. (A) The apoptosis of U87-MOCK and U87-PDCD5 cells when treated with different concentrations of CDDP for 48 h (Hoechst, 200× ). U87-PDCD5 cells appeared conspicuous nuclear condensation and underwent more apoptosis than U87-MOCK cells in the present of CDDP. (B) The late apoptosis of U87-MOCK and U87-PDCD5 cells after treated with CDDP for 48 h by TUNEL assay. U87-PDCD5 cells showed more apoptosis than U87- MOCK cells at 25 μg/ml CDDP.
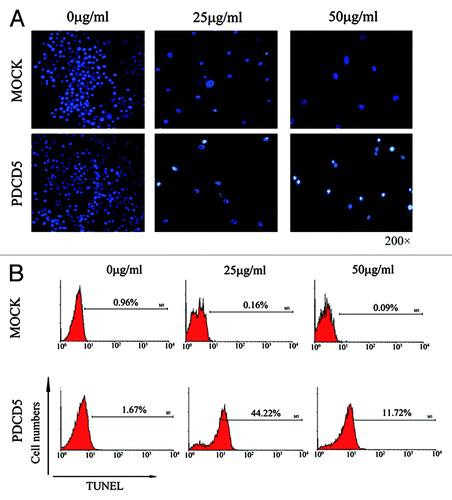
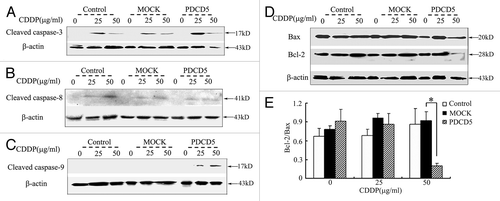
Figure 6. The effect of exogenous overexpression of PDCD5 on apoptosis related proteins. (A) The expression level of cleaved caspase-3 was examined by western blot. Expression of cleaved caspase-3 was significantly increased in U87-PDCD5 cells than those of two control cells (Control and MOCK groups) after treatment with CDDP for 48 h. (B) The expression level of cleaved caspase-8 was examined by western blot. There was no significant difference about cleaved caspase-8 expression between U87-PDCD5 cells and control cells after CDDP treatment. (C) The expression level of cleaved caspase-9 was examined by western blot. Expression of cleaved caspase-9 was significantly increased in U87-PDCD5 cells than those of two control cells after treatment with CDDP for 48 h. (D) The protein of Bcl-2 and Bax in U87-MOCK and U87-PDCD5 cells after treated with CDDP (0, 25, 50μg/ml) for 48 h was examined by western blot. (E) The expression of Bcl-2 and Bax was analyzed by normalized to β-actin. The ratio of Bcl-2/Bax in U87-PDCD5 cells decreased after 50 μg/ml CDDP treatment (*p < 0.05). Control: untransfected cells; MOCK: transfected cells with plasmids pcDNA3.1; PDCD5: transfected cells with plasmids pcDNA3.1 containing full length of PDCD5 gene.