Figures & data
Table 1. Demographics and clinical characteristics of 92 newly diagnostic ALL patients
Figure 1. Kaplan-Meier curves of overall survival for B-ALL patients. Newly diagnosed B-ALL patients were stratified in two groups according to HIF-1α protein expression level: highest level (quartile 4) and lower level (quartile 1–3).
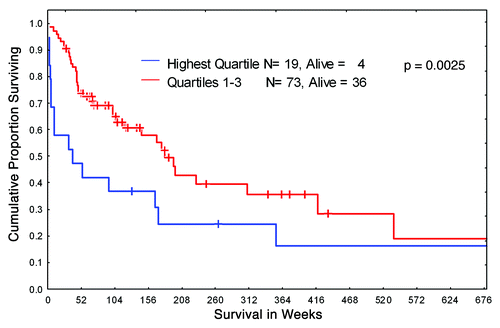
Figure 2. HIF-1α and cytotoxic effects of chemotherapy at 1% O2. (A) REH cells were transfected with a scramble or HIF-1a LNA, treated with the indicated chemotherapy agent, and incubated at 1% O2 for 48 h. Western blot showing HIF-1α knockdown achieved with HIF-1α LNA but not with the scramble LNA. UNT, untreated; Vnc, vincristine; MTX, methotrexate; DOX, doxorubicin; AnnV, Annexin V; Scr, scrambled LNA. (B, C) REH cells or NALM6 cells were treated with 100µM DMOG (B) or infected with empty vector or HIF-1α lentivirus and induced with 1µg/ml Doxocycline (REH, C). Western blots show expression of HIF-1α in DMOG (96hr treatment) or Doxocyline treated cells. After inducing HIF-1α expression at 21%O2, cells were treated with chemotherapy (VCR or ETO). After 72 h, effects on cell growth and apoptosis induction were determined by FACS. Growth inhibition was calculated for each group (DMSO, DMOG, Scr or HIF-1α) as the percentage relative to the untreated control. *p < 0.05; ** p < 0.01.
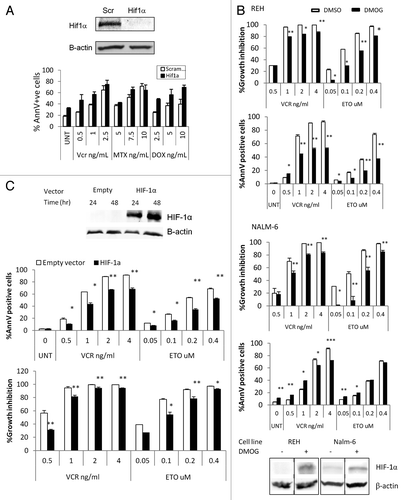
Table 2. Clinical data for ALL patients whose samples were used for in vitro studies
Figure 3. A and B. Activation of HIF-1α, pAKT and pERK protein expression in ALL cells co-cultured with bone marrow-derived MSC under hypoxia. REH cells and primary ALL blasts were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions for the indicated time periods (with or without MSCs). Expression of HIF-1α (A), pAKT Ser473 and pERK proteins (B) was analyzed by immunoblotting. (C)Time-course of HIF-1α expression for six ALL xenograft lines. Cells were cultured with and without MS-5 and harvested at the indicated time points. EF1α was used as an internal control as described in the methods. Each fold change in relative HIF-1α mRNA expression levels was calculated based on their non-MS5-cultured counterpart. Results are the mean ± SE of at least three separate experiments.
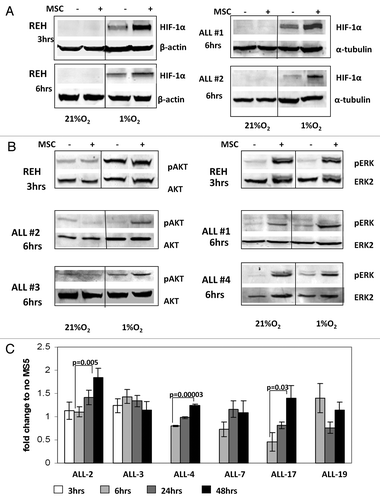
Table 3. Clinical data from childhood ALL patients’ xenograft samples
Figure 4. Inhibition of mTOR downregulates HIF-1α, pS6K and pAKT expression in leukemic cells co-cultured with MSC under hypoxia. REH cells (A, B) or primary ALL cells (#5, B) were cultured under the indicated oxygen levels in the presence or absence of 20nM everolimus or PD98059 10µM (with or without MSCs). Expression of HIF-1α, pS6K/S6K, pAKT ser473/AKT and pERK/ERK2 proteins was determined by Western Blot.
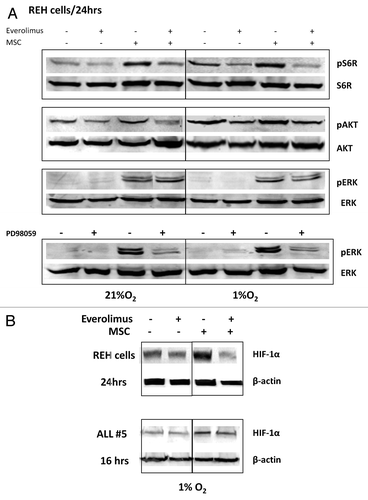
Figure 5. Everolimus decreases glucose uptake and reduces lactic acid production in leukemic cells. REH and primary ALL cells were grown under normoxic (21% O2) or hypoxic (1% O2) conditions and treated with 20nM everolimus for the indicated time period. (A) Glucose uptake was measured by Flow Cytometry. The results are presented as mean fluorescent intensity (MFI). (B) Lactic acid (LA) concentration was measured in the aliquots collected from the medium using Accutrend Lactate device (Roche). The data was expressed as pM per cell. Left, REH; right, averaged data from 4 primary ALL samples (#12–15, Table S1). (C) REH and primary ALL cells (#2, Table S1) were cultured with or without MSC under normoxic (21% O2) or hypoxic (1% O2) conditions in the presence or absence of 20nM everolimus for 24hrs. HKII protein expression was examined by Western Blot. Expression of β-actin was used as a loading control.
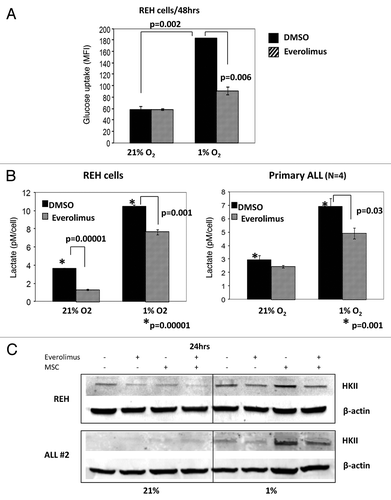
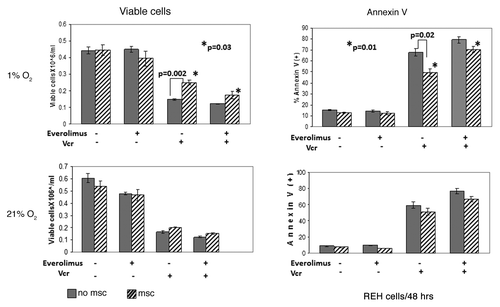
Figure 6. Inhibition of mTOR signaling sensitized leukemic cells to chemotherapy under hypoxic conditions mimicking BM microenvironment. Exponentially growing REH cells were cultured with or without MSC for 48 h under normoxia (21% O2) and then were grown under normoxic (21% O2) or hypoxic (1% O2) conditions in the presence or absence of 20nM everolimus and/or 1ng/ml Vcr. After 48 h, effects on cell growth and apoptosis induction were determined by viable cell count and annexin V flow cytometry. *p = 0.01–0.03