Figures & data
Figure 1. C1B peptides significantly inhibit anchorage-independent growth of human colon cancer cells. Colonies over 50µm diameter were counted by an automated colony counter as described in the Materials and Methods. COLO205 cells (2 × 104) were cultured in soft agar in the presence or absence of various concentrations of C1B5 (A) or C1B1 peptide (B) for 7 d. (C) Colony size was also significantly smaller in the C1B peptide-treated group. Original magnification is x100. (D) C1B5 peptide (1 μΜ) effectively attenuated colony growth in two other human colon cancer cell lines, SW620 and Caco-2. Data are expressed as mean ± SE. Asterisks indicate significantly different values (p < 0.05) when compared with the control.
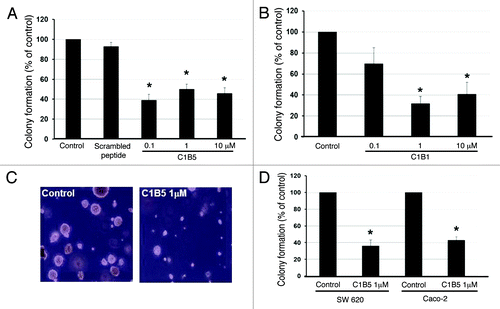
Figure 2. C1B5 peptide did not affect the growth of RIE-1 normal intestinal epithelial cells or COLO 205 cells in 2D culture. Cells were cultured in the absence (white bars) or presence of 0.1 μM (gray bars) or 1 μM (black bars) C1B5 peptide for various time periods as indicated in the figure. Dead cells were stained with trypan blue and the cell numbers were analyzed using a hemocytometer. Data are expressed as mean ± SE.
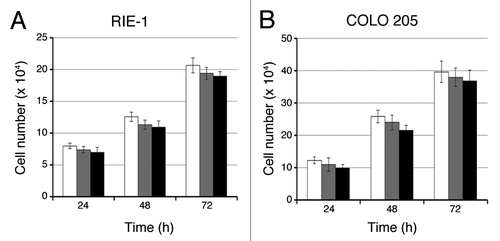
Figure 3. Inhibition of anchorage independent growth by C1B5 peptide is reversed by PCKα/β-specific inhibitor. As in , a soft agar assay was used and COLO205 colonies were counted with an automated colony counter. Pretreatment with the PKCα/β- inhibitor, Ro-32-0432 (1 µM), counteracted C1B5-induced colony growth attenuation. These data are representative of two independent analyses with triplicate determinations. Data are expressed as mean ± SE. An asterisk indicates significantly different value (p < 0.05) when compared with the control.
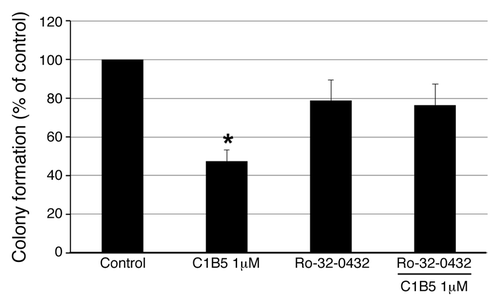
Figure 4. C1B5 peptide treatment significantly increased the G2 population (panels A and B), both early and late-stage apoptosis (panels C and D), and cleaved caspase 3 and phosphorylated p53 of COLO205 cells (panels E and F). COLO205 cells were incubated for 72 h with 1 μM C1B5 peptide. Cells were dispersed by trypsinization and incubated with either propidium iodide for cell cycle analysis or annexin V-FITC for apoptosis analysis by flow cytometry as described in Materials and Methods. These data are representative of two independent analyses with triplicate determinations. Both early apoptosis (as shown in lower right quadrant) and late apoptosis (upper right quadrant) increased significantly with the addition of C1B5 (panel D). Samples for the Western blot analysis were collected either at 48 or 72 h. Sample preparation and Western blot analysis were performed three times with duplicate determinations. The pictures in panel E represent typical blotting results. An asterisk indicates a significantly different value (p < 0.05) when compared with the control.
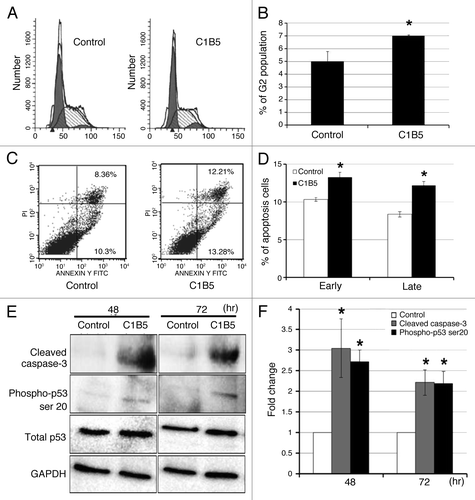
Figure 5. The effect of C1B5 treatment on the expression of various PKC isozymes, PKC phosphorylation, cleaved caspase-3, total p53, and phosphorylated p53 in COLO205 was analyzed by Western blot analysis. C1B5 significantly decreased levels of phosphorylated PKCα/βII after 30 min of exposure (Panels A and D). In contrast, the levels of non-phosphorylated PKCα and βwere increased by the C1B5 treatment (Panels A, B and C). The level of cleaved caspase-3 was significantly increased at 6 h (Panels A and E). The expression levels of total p53 and phosphorylated p53 at serine 20 were significantly increased after C1B5 treatment (Panels A, F, and G). Samples were prepared and subjected to Western blot analysis as described in Materials and Methods. Sample preparation and Western blot analysis were performed three times with duplicate determinations. The pictures in Panel A represent typical blotting results. Asterisks indicate significantly different values (p < 0.05) when compared with the 0 h time point.
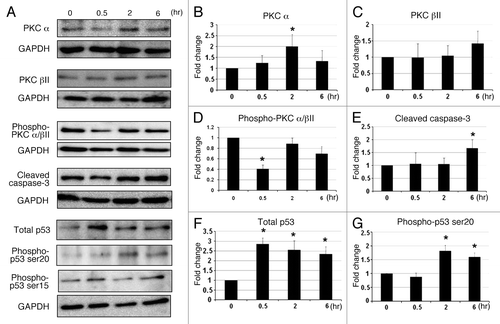
Figure 6. Intratumoral injection of C1B5 peptide significantly attenuated the growth of COLO205 subcutaneous xenografts in SCID mice (n = 5, panels A and B). Histologically, the number of mitotic figures was significantly smaller in the C1B5 treated group tumors than in the scrambled peptide and PBS control group tumors (panels C and D). The H&E sections show the mitotic figures (arrows, panel C, 400× magnification). In this experiment, COLO205 cells were inoculated subcutaneously to both flanks of SCID mice at 1 × 107 cells per site. When tumors reached an average volume of 150 mm3, C1B5, scrambled peptide or PBS was injected into the tumors. The treatment was initiated at day 22 after tumor cell inoculation and repeated every 3 d for 15 d. Mice were sacrificed 3 d after the final treatment. The number of mitotic figures in 10 randomly selected fields at high power field (400x) was counted and the mean number of mitotic figures was expressed. Data are expressed as mean ± SE. Asterisks indicate significantly different values (p < 0.05) when compared with the PBS control.
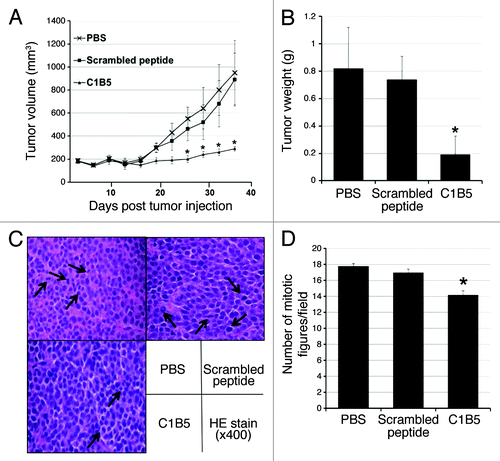
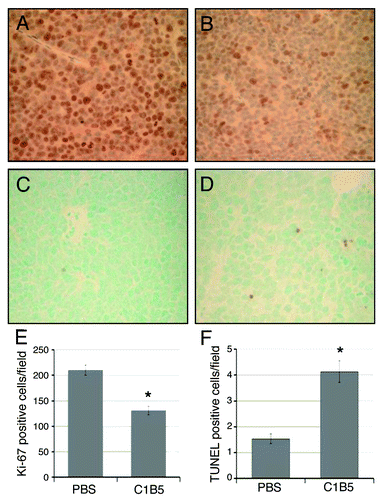
Figure 7. Immunohistochemical analysis of cell proliferation (panels A, B and E) and apoptosis (panels C, D, and F) in COLO205 subcutaneous xenografts in SCID mice treated intratumorally with either PBS (panels A and C) or C1B5 (panels B and D). Xenografts were collected as described in the Methods section. Cell proliferation in COLO205 xenografts was analyzed by counting the number of Ki-67 positive cells (panels A and B). Apoptosis was analyzed by counting TUNEL positive cells in the tumors (panels C and D). The average numbers of Ki-67 positive cells (E) and TUNEL positive cells (F) per high power field (40x) were determined by analyzing ten fields in each treatment group and are expressed in the bar graph. The original magnification of panels A-D was 40x. Asterisks indicate significantly different values (p < 0.05) when compared with the PBS control.