Figures & data
Figure 1. (A)(a) Chemical structures of compounds used in this study. (b) Whole plant extracts of Nigella sativa were used in this study. (B) Cytotoxic effect of ETP alone (■) or in the presence of (a) NS (▲) or (b) taurine (▲) on MCF-7, HepG2, U251, HeLa and HCT116 cell lines. Tumor cell lines were treated with different concentrations of ETP ± 50 µg/ml NS or ± 100 µg/ml taurine. Cell counts were performed after 48 h of exposure to drugs. Each point is the mean ± SEM of three separate experiments.
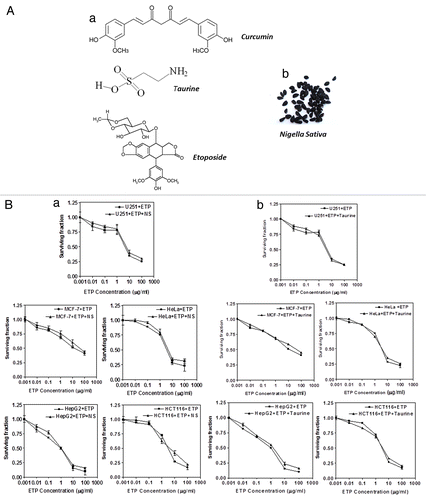
Figure 2. Cell survival after treatment of tumor cell lines with ETP and/or CUR. MCF-7, HepG2, HCT116, HeLa and U215 cells were cultured in micro-well plates. Twenty-four hours later, the cells were treated with different concentrations of ETP and/or CUR. After 48 h of exposure to the drugs, the cells were fixed, stained with Sulphorhodamine-B (SRB) and the optical density was measured spectrophotometrically at 564 nm. Survival fraction = OD564 of drug treated sample/OD564 of untreated control sample. (a) Statistical significance compared with ETP alone using an unpaired t-test, p < 0.01. It should be noted that, although the effect of the combination may be significantly high er than ETP alone but it is still lower than the sum of the effects of either agent alone which indicates antagonism
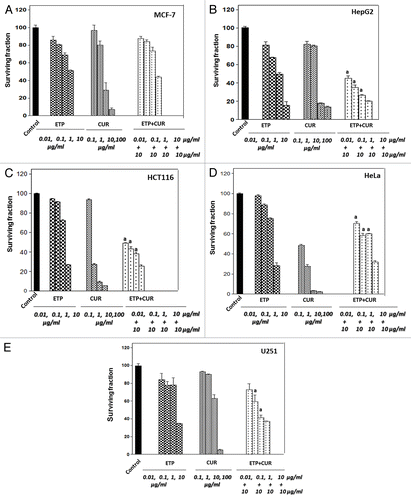
Table 1. Effect of CUR on ETP cytotoxicity in MCF-7, HepG2, HeLa, HCT116 and U251 cells
Figure 3. Formation of γ-H2AX foci after treatment with ETP and/or CUR in HeLa cells. (A) An example of γ-H2AX foci (white) and DNA (green) staining with and without treatment. (B) Quantification of γ-H2AX foci, MCF-7, HepG2, HCT116 and HeLa Cells were treated with ETP (1 µg/ml) and/or CUR (10 µg/ml) for 24 h and subjected to immunofluorescence detection of γ-H2AX foci assay. Results are the average and standard deviation of three repeats; on each occasion at least 50 cells were counted for each condition. (C) HeLa cells were treated with 10µg/ml CUR for 48 h, and morphological changes were observed using an inverted microscope (100×). (a) Significantly different from ETP alone at p < 0.05. It should be noted that, although the effect of the combination may be significantly high er than ETP alone but it is still lower than the sum of the effects of either agent alone which indicates antagonism
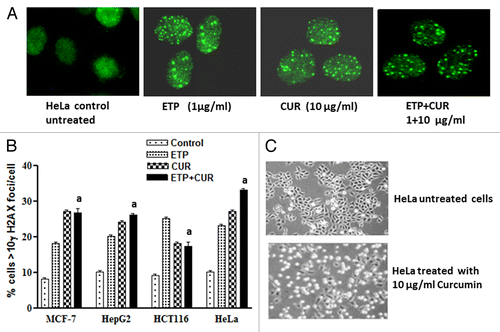
Figure 4. Induction and repair of DNA double-strand breaks (DSB) induced by ETP and/or CUR in human tumor cell lines. (A) Ethidium bromide staining of DNA double-strand breaks visualized with constant field gel electrophoresis (CFGE) after 48 h treatment with increasing doses of CUR in HeLa cells. (B) Induction of DSBs measured by CFGE. MCF-7, HepG2, HCT116 and HeLa cells were treated with ETP (1 µg/ml) and/or CUR (10–50 µg/ml) for 48 h, immediately followed by the measurement of DSB by CFGE. Constant field gel electrophoresis was run at 0.6 V cm−1 for 30 h. The fluorescence intensity of each band in each lane was recorded with a gel documentation system and video camera. (C) The band intensities in ethidium bromide-stained gels were quantified, and the fraction of DNA released (FDR) at each drug concentration was calculated. The FDR released into the gel, corresponding to fragmented DNA, was calculated according to the following equation: FDR = FDR rel / (FDR plug +FDR rel). FDR rel: the fraction of DNA released outside the well. FDR plug: the fraction of DNA remaining in the well. Data represent the results of three independent experiments. (a) Significantly different from ETP alone at p < 0.05.
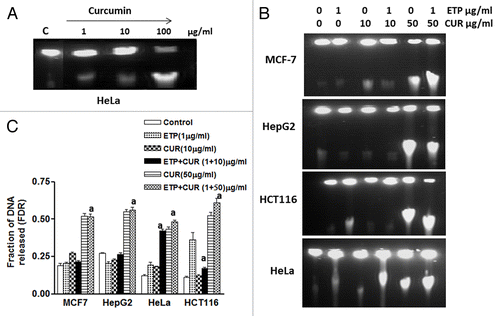
Figure 5. Cell cycle distribution analysis of the human tumor cell lines treated with ETP and/or CUR. (A) MCF-7, (B) HepG2, (C) HCT116 and (D) HeLa cells were treated with ETP (1 µg/ml) and/or CUR (10 µg/ml) for 48 h. The cells (control and treated) were collected by trypsinisation, washed, incubated with RNase and treated with propidium iodide; the DNA content was then measured by flow cytometry. ETP or CUR treatment blocked the cells at the intra-S-phase checkpoint, whereas combined treatment (ETP + CUR) arrested the tumor cells in the S and/or G2/M phases. The percentage of cells in the subG1, G1, S and G2/M phases are detailed in graphs on the right panel and in . Data refer to three experiments.
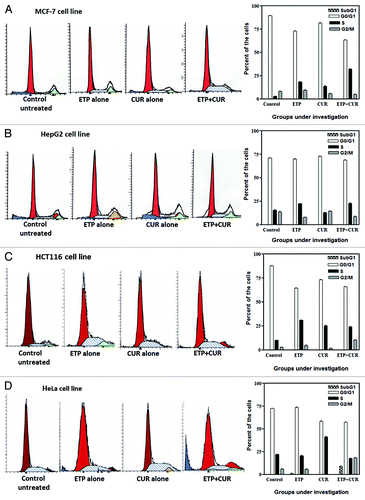
Table 2. Cell cycle modifications induced by ETP, CUR and their combination in MCF-7, HepG2, HCT116 and HeLa cells after 48 h of treatment
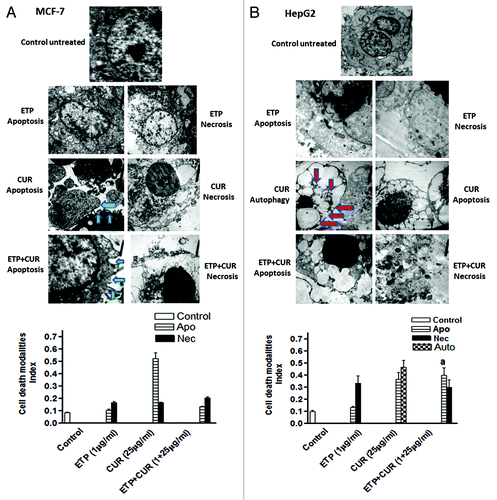
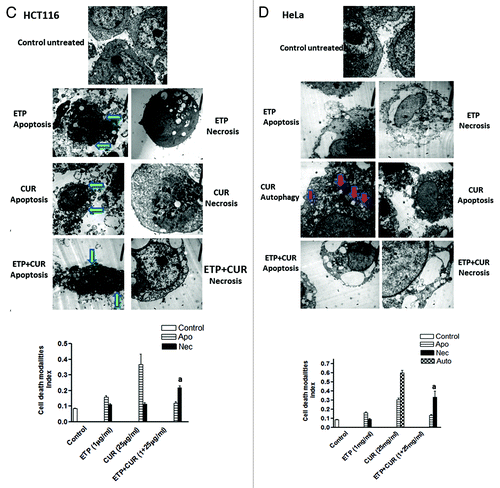
Figure 6A and B. Representative electron micrographs of (A) MCF-7, (B) HepG2, (C) HCT116 and (D) HeLa cells treated with DMSO (control) or ETP (1 µg/ml) and/or CUR (25 µg/ml) for 24 h. Different modalities of programmed cell death (PCD) were observed after treatment with ETP and CUR, alone and in combination. CUR induced autophagy, apoptosis and necrosis in tumor cell lines. HepG2 and HeLa cells showed autophagic vesicles after treatment with 25 µg/ml CUR. Arrows indicate autophagic vacuoles. MCF-7 and HCT116 cells showed signs of apoptosis, including chromatin condensation, cell shrinkage, cytoplasmic blebbing and fragmentation. ETP alone induced apoptosis and necrosis. Cotreatment with ETP and CUR caused apoptosis and necrosis. Bars represent mean ± SD in each treatment group. The mean density for autophagic, apoptotic and necrotic was determined by counting the number of cells showing these modes of cell death in both ETP and /or CUR-treated cells and control cells in ten low power fields (2000X) of each section in a blinded fashion. (a) Significantly different from ETP alone at p < 0.05. It should be noted that, although the effect of the combination may be significantly high er than ETP alone but it is still lower than the sum of the effects of either agent alone which indicates antagonism.