Figures & data
Figure 1. VE-821 radiosensitizes pancreatic tumor cells. (A) Western blot analysis of Chk1 inhibition. Cells were treated with 100 nM gemcitabine and 1 µM VE-821 1 h prior to irradiation at 6 Gy as indicated in the graphical representation. Drugs were left for the duration of the experiment and cells were lysed at 2 h post-irradiation and subjected to western blot analysis. (B) Effect of VE-821 on cell viability of pancreatic cancer cells with and without radiation treatment. PSN-1, PANC-1, and MiaPaCa-2 cells were treated with increasing concentrations of VE-821 for 72 h. Cells were irradiated at 4Gy 1h after VE-821 addition. Cell viability was measured after 10 d and shown as normalized to DMSO-treated cells. (C) Scheduling of VE-821 affects radiosensitivity. PSN-1 cells were plated as single cells, treated with 1 µM VE-821 at different time points in relation to 4 Gy irradiation and assessed for colony formation after 10 d as indicated in the graphical representation. The survival fraction at 4 Gy for each of the treatment schedules was determined by taking into account the relevant plating efficiency of unirradiated cells (see Fig. S1B). (D) Clonogenic survival of pancreatic cancer cells, MiaPaCa-2, PSN-1, PANC-1 and primary cancer cells PancM in response to irradiation and VE-821 treatment. Cells were treated according to the 72h VE-821 treatment regime described in (C) with 1 µM VE-821 added at 1 h prior to irradiation and removed 72 h post-irradiation and colony-forming ability being assessed after 10 to 21 d. N = 3; *p < 0.05; **p < 0.01 over DMSO-treated control.
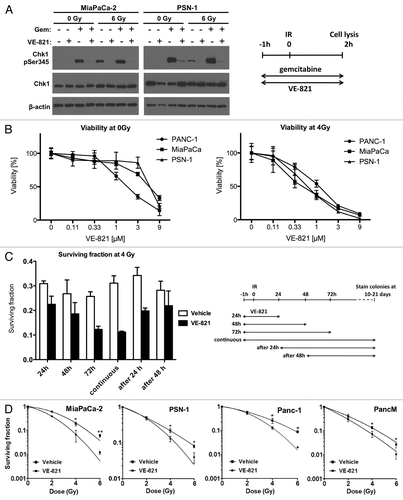
Figure 2. VE-821 radiosensitizes pancreatic tumor cells under hypoxic conditions. (A) Clonogenic survival curves of cells treated with 1 µM VE-821 and irradiation under hypoxic conditions as indicated in the graphical representation. Plated cells were transferred to hypoxia (0.5% O2) and acclimatized for 6 h. VE-821 (1 µM) was then added at 1h prior to irradiation and left for 72 h upon which the medium was replaced. Cells were transferred to normoxia at 1 h post-irradiation. (B) Bar graph representation of clonogenic survival of cells after irradiation with 6 Gy and treatment with 1 µM VE-821 in oxic and hypoxic (0.5% O2) conditions, as shown above and in . N = 3; *p < 0.05; **p < 0.01; ***p < 0.001 over DMSO-treated control.
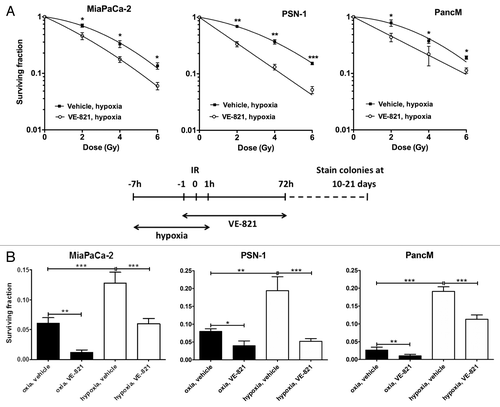
Figure 3. VE-821 sensitizes pancreatic cancer cells to gemcitabine treatment. (A) Clonogenic survival of cells treated with gemcitabine and 1 µM VE-821. Cells were treated with increasing concentrations of gemcitabine for 24 h followed by 72 h treatment of 1 µM VE-821. Colony forming ability was assessed after 10 to 21 d (see graphical representation in panel E). (B) Clonogenic survival of cells treated with gemcitabine in hypoxia. Plated cells were transferred to hypoxia (0.5% O2) and acclimatized for 6 h. Cells were then treated with increasing concentrations of gemcitabine for 24 h followed by 72 h treatment of 1 µM VE-821. Hypoxic cells were transferred to normoxia 1 h after VE-821 addition. (C) Bar graph representation of clonogenic survival after treatment with 20 nM gemcitabine and VE-821 in oxic and hypoxic (0.5% O2) conditions, shown in (A and B). (D) Clonogenic survival of cells treated with gemcitabine and irradiation. PSN-1 and MiaPaCa-2 cells were treated with 5 nM or 10 nM gemcitabine, respectively, for 24 h, medium was then replaced and 1 µM VE-821 was added from 1 h prior to 72 h post 4 Gy irradiation. Colony forming ability was assessed after 10 to 21 d. (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 over DMSO-treated control. (E) Graphical representation for the treatment regimes used in panels [A, B, (C) and D].
![Figure 3. VE-821 sensitizes pancreatic cancer cells to gemcitabine treatment. (A) Clonogenic survival of cells treated with gemcitabine and 1 µM VE-821. Cells were treated with increasing concentrations of gemcitabine for 24 h followed by 72 h treatment of 1 µM VE-821. Colony forming ability was assessed after 10 to 21 d (see graphical representation in panel E). (B) Clonogenic survival of cells treated with gemcitabine in hypoxia. Plated cells were transferred to hypoxia (0.5% O2) and acclimatized for 6 h. Cells were then treated with increasing concentrations of gemcitabine for 24 h followed by 72 h treatment of 1 µM VE-821. Hypoxic cells were transferred to normoxia 1 h after VE-821 addition. (C) Bar graph representation of clonogenic survival after treatment with 20 nM gemcitabine and VE-821 in oxic and hypoxic (0.5% O2) conditions, shown in (A and B). (D) Clonogenic survival of cells treated with gemcitabine and irradiation. PSN-1 and MiaPaCa-2 cells were treated with 5 nM or 10 nM gemcitabine, respectively, for 24 h, medium was then replaced and 1 µM VE-821 was added from 1 h prior to 72 h post 4 Gy irradiation. Colony forming ability was assessed after 10 to 21 d. (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 over DMSO-treated control. (E) Graphical representation for the treatment regimes used in panels [A, B, (C) and D].](/cms/asset/3a353264-6b8c-4ca3-81a1-8ba14cb5474f/kcbt_a_10921093_f0003.gif)
Figure 4. VE-821 perturbs the irradiation-induced cell cycle checkpoint in pancreatic cancer cells. VE-821 (1 µM) was added 1 h prior to 6 Gy irradiation and left for the duration of the experiment. Cells were lifted and fixed at 12 h or 24 h after irradiation, stained with propidium iodide and analyzed for cell cycle distribution by flow cytometry. Bar graphs represent mean +/− SEM (n = 6). Individual DNA histograms of representative samples are shown in Figure S2A.
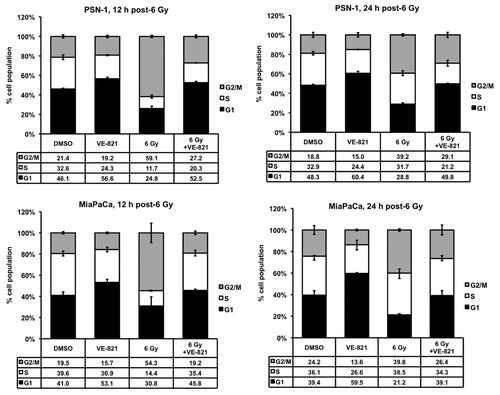
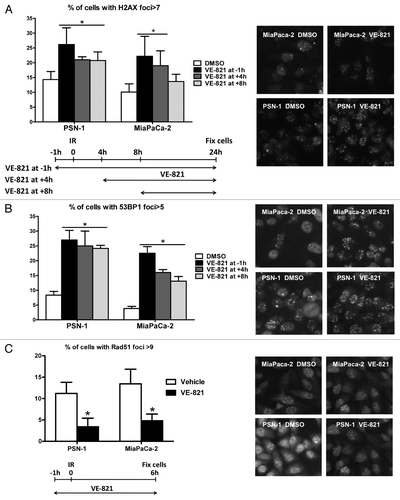
Figure 5. VE-821 increases 53BP1 and γH2AX foci number and reduces Rad51 foci formation. Cells were treated with 1 µM VE-821 at various time points in relation to 6 Gy irradiation, as indicated in the legends and the graphical representation, and fixed at 24 h post-irradiation. Subsequently, cells were stained for (A) γH2AX and (B) 53BP1 foci and the percentage of cells with more than 7 and 5 foci per cell was quantitated, respectively. (C) For analyzing Rad51 foci formation, cells were fixed at 6 h post-irradiation as indicated in the graphical representation and the percentage of cells with more than 9 foci per cell was quantitated. Representative images are shown on the right. N = 4; *p < 0.05 over DMSO treated controls.