Figures & data
Figure 1. Activation of Fas/Fas-L pathway in melanoma cells. DM6 or A2058 melanoma cells were cultured in absence or presence of agonistic anti-Fas antibody, CH-11, at a concentration of 1 µg/mL for 72 h. (A) western blot and bar graphs of procaspase-8 expression after CH-11 treatment. Bars represent mean ± SEM expressed as percentage of change from three separate experiments, (*p < 0.05) decrease in the level of expression. (B) MTT assay was used to determine cell survival. Treatment of A2058 cells with CH-11 induced significant cytotoxicity compared with non-treated cells. Each bar represents the mean ± SD of three independent experiments (*p < 0.05). (C) Annexin V staining was used to determine the percentage of apoptosis. Cells were stained with annexin V-PE and 7-AAD 72 h after treatment. Cells positive for annexin V-PE and 7-AAD staining were analyzed by FACScan flow cytometer with FlowJo software. One representative experiment is shown from three performed.
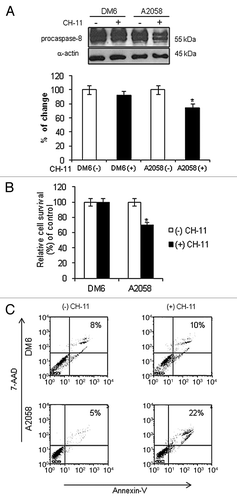
Figure 2. FKHRL1/TM induces cytotoxicity, caspase pathway activation, and apoptosis. A2058 and DM6 melanoma cell lines were infected at increasing doses (0–100 MOI) of either Ad-LacZ or Ad-FKHRL1/TM. At 72 h cells were analyzed by MTT, and western blot assays. (A) MTT assay was used to determine cell survival comparing Ad-LacZ with Ad-FKHRL1/TM treatments. Each point represents the mean ± SD of three independent experiments (p < 0.05). (B) Expression of FKHRL1/TM, proenzyme CPP32/caspase-3 (CPP32), PARP and cleaved components of PARP (PARP/C) were detected by western blot. α-Actin was used to demonstrate equal loading for each lane. (C) A2058 and DM6 melanoma cell lines were not infected (Mock) or infected with either Ad-LacZ or Ad-FKHRL1/TM at an MOI of 100. Three days post-infection the percentages of apoptosis were determined by annexin V staining, and analysis by FACScan flow cytometer with FlowJo software. Similar results were obtained in three independent experiments. A representative experiment is shown.
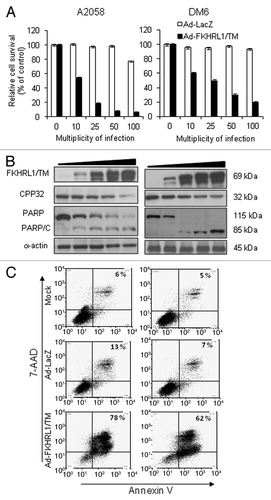
Figure 3. Activation of Fas/Fas-L pathway by FKHRL1/TM in melanoma cells. DM6 or A2058 cells were infected with either Ad-LacZ or Ad-FKHRL1/TM at an MOI of 100 or not infected (Mock); cells were harvested at 24, 48, and 72 h post-infection. (A) Western blot and bar graphs of procaspase-8 expression after adenovirus infection. Bars represent mean ± SEM expressed as percentage of change from three separate experiments, (*p < 0.05) decrease in the level of expression. (B) Western blot and bar graphs of Fas-L expression at 72 h after adenovirus infection. Bars represent mean ± SEM expressed as percentage of change from 3 separate experiments, (*p < 0.05) increase in the level of expression. α-Actin was used to demonstrate equal loading for each lane.
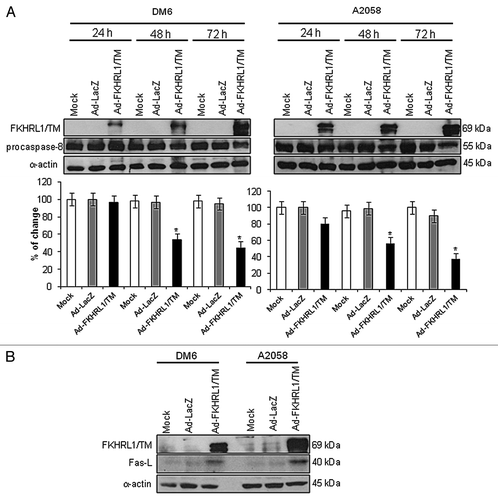
Figure 4. Inhibition of Fas-L-mediated cell death. (A) DM6 or A2058 cells were cultured with increasing concentrations of Fas/Fas-L antagonist Kp7-6 followed by infection with either Ad-LacZ or Ad-FKHRL1/TM at an MOI of 50. At 72 h an MTT assay was performed to determine cell survival. Ad-FKHRL1/TM alone was compared with Ad-FKHRL1/TM in presence of Kp7-6. Each point represents the mean ± SD of three independent experiments (*p < 0.05). (B) A2058 cells were cultured in absence or presence of Fas/Fas-L antagonist Kp7-6 at 10 µg/mL followed by mock infection or infection with either Ad-LacZ or Ad-FKHRL1/TM at an MOI of 100. Western blot and bar graphs of procaspase-8 expression after adenovirus infection. Bars represent mean ± SEM expressed as percentage of change from 3 separate experiments, (*p < 0.05) decrease in the level of expression. α-Actin was used to demonstrate equal loading for each lane. FKHRL1/TM expression was also confirmed.
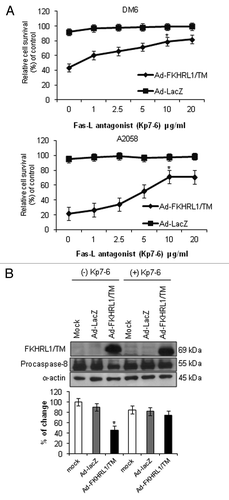
Figure 5. FKHRL1/TM sensitizes melanoma cells to CH-11 mediated-apoptosis and downregulates IAP family members. (A) A2058 or DM6 cells were infected with either Ad-LacZ or Ad-FKHRL1/TM at an MOI of 10 +/− treatment with CH-11 at 1 µg/mL. Annexin V staining was used to determine the percentage of apoptosis at 72 h after treatment by FACScan flow cytometer with FlowJo software. Ad-FKHRL1/TM infection alone was compared with Ad-FKHRL1/TM in combination with CH-11. Each bar represents the mean ± SD of three independent experiments (*p < 0.05). (B) A2058 or DM6 cells were no infected (mock) or infected with either Ad-LacZ or Ad-FKHRL1/TM at an MOI of 100 at 72 h post-infection expression of X-IAP, C-IAP-1, or C-IAP-2 were analyzed, a representative experiment is shown from three performed.
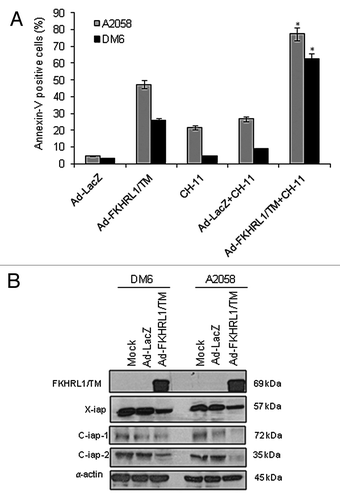
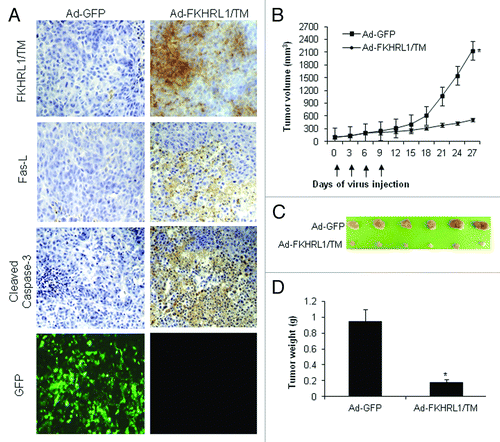
Figure 6. Immunohistochemistry and antitumor effect of treatment with adenovirus expressing FKHRL1/TM. After subcutaneous administration of 5 × 106 A2058 cells, mice with palpable tumors were randomized to receive a local injection of Ad-GFP or Ad-FKHRL1/TM on days 0, 3, 6, and 9 (vertical arrows) at a concentration of 1 × 109 pfu (plaque forming units). (A) Immunohistochemistry of A2058 melanoma tumors at 24 h after last treatment with Ad-GFP or Ad-FKHRL1/TM. GFP expression was visualized by fluorescence microscopy. FKHRL1/TM, Fas-L, and cleaved caspase-3 expressions were detected by immunohistochemistry. (B) Tumor volume was plotted over time. Volume (V) was determined by V = (L × W 2)/2 of tumor measured using calipers. (C) On day 27, tumors were excised and photographed. The relative size of tumors is shown. (D) Tumors were also weighed on day 27. The results are expressed as the mean ± SEM for each treatment group. The differences in tumor size and weight in animals treated with Ad-FKHRL1/TM were statistically significant on day 27 compared with Ad-GFP control (*p < 0.05).