Figures & data
Figure 1. Diarrhea onset and group proportions in lapatinib dose-finding experiment. Survival curves show days to first diarrhea in rats treated with different doses of lapatinib (n = 30).
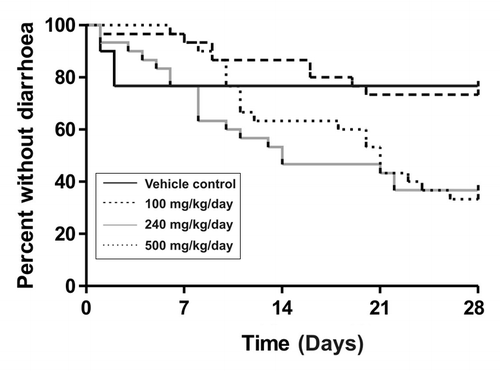
Figure 2. Daily bodyweight in combination treatment experiment. Graph shows the daily weight gain or loss of rats over 5 weeks in each group. Data presented is mean ± SEM (n = 24 in the control arm and n = 48 in the treatment arms).
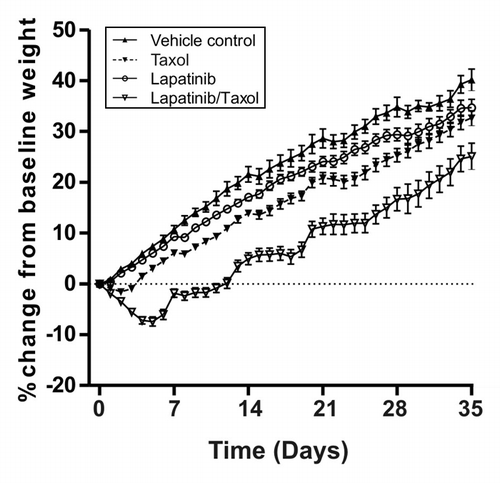
Figure 3. Diarrhea onset and severity in combination treatment experiment. (A) Survival curves show days to first diarrhea in rats across the different groups Rats treated with combined lapatinib and paclitaxel (Taxol) had significantly earlier diarrhea (p = 0.034, Log Rank Test) compared with lapatinib only treated rats. (B) Graph shows proportions of diarrhea severity across the different groups (n = 24 in control arm, n = 48 in treatment arms). Rats treated with combined lapatinib and paclitaxel (Taxol) had a significantly higher proportion of severe diarrhea (p = 0.0002, Chi Squared Test) compared with lapatinib only treated rats.
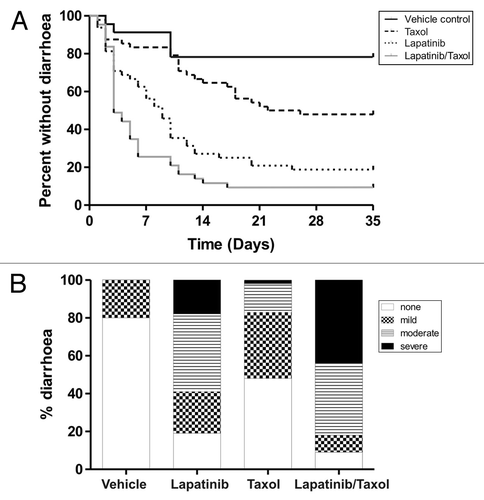
Figure 4. Organ weights in combination treatment experiment. Graphs show organ weights as a percentage of total body weight across the different groups. Data presented is mean ± SD (n = 4 in control arm and n = 8 in treatment arms). Relative small intestinal was significantly higher between weeks 1 and 5 in rats treated with combined lapatinib and paclitaxel (Taxol) compared with all other groups (p < 0.001, Two-way ANOVA with Bonferroni post-test). Relative large intestinal weight was significantly increased in the combined lapatinib and paclitaxel (Taxol) rats at week 2 compared with lapatinib only treated rats (p < 0.05, Two-way ANOVA with Bonferroni post-test). Relative stomach weight was significantly increased at week 3 in the combined lapatinib and paclitaxel (Taxol) group compared with lapatinib only treated and paclitaxel (Taxol) only treated groups (p < 0.01, Two-way ANOVA with Bonferroni post-test). Relative spleen weight was significantly increased at week 3 in the combined lapatinib and paclitaxel (Taxol) group compared with lapatinib only treated rats (p < 0.05, Two-way ANOVA with Bonferroni post-test) and at week 4 compared with vehicle controls (p < 0.05, Two-way ANOVA with Bonferroni post-test).
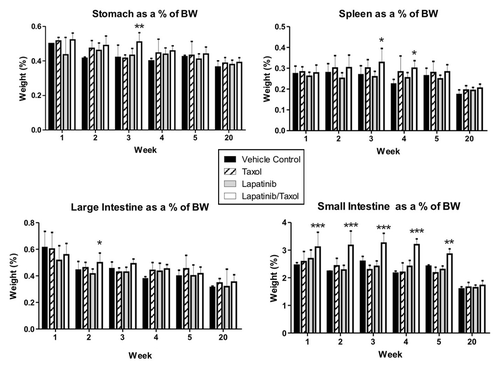
Figure 5. Photomicrographs of intestinal histopathology. Colon and jejunal tissue sections from each rat were stained with H&E for routine histopathological analysis. Representative images for each group at week 4 of treatment are presented. Original magnification × 100. Scale bar = 100 μm.
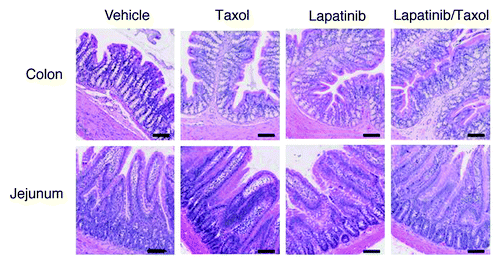
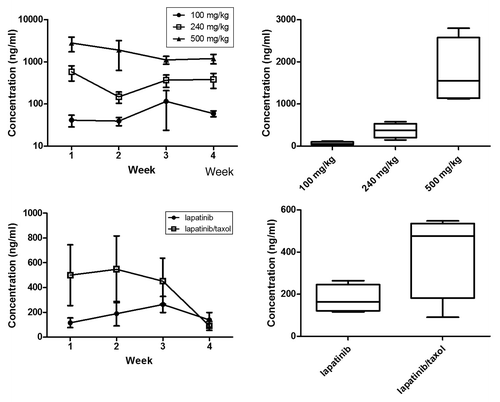
Figure 6. Serum lapatinib concentration. (A) Serum lapatinib concentrations over time in the lapatinib dose finding experiment (n = 6). (B) Overall group mean serum lapatinib concentrations in the lapatinib dose finding experiment (n = 6, data combined over 4 weeks). (C) Serum lapatinib concentrations over time in the combination treatment experiment (n = 8). (D) Overall group mean serum lapatinib concentrations in the combination treatment experiment (n = 8, data combined over 4 weeks). Rats in the combination group had significantly higher mean lapatinib concentrations compared with lapatinib only treated rats (p = 0.027, Repeated measures Two-way ANOVA). Data presented is mean ± SEM.