Figures & data
Figure 1. (A) Protective effect of 10 mM alanyl-glutamine (NFV+AQ) supplementation on IEC-6 cell migration following 1 h incubation with 70 μg/mL of nelfinavir (NFV) at 12 and 24 h. After reaching confluency, IEC-6 monolayers were scratched, wells were incubated with 70 μg/mL of NFV for 1h and washed with media without glutamine, followed by incubation with 10mM of AQ. The bars represent means ± SE for the number of migrating cells per square millimeter of scraped area. *p < 0.05, compared with control group with media without glutamine, by student’s unpaired t test. #p < 0.05, compared with group with NFV, by student’s unpaired t test. (B) Representative images of migration of IEC-6 cells at 24 h from the control group, NFV group, and AQ supplemented group, following NFV exposure for 1 h. Diagram shows scraping area with grid (each square = 0.1 mm2), overlapping the column of the farthest migration. The IEC-6 cells were tracked by traced dots for counting. The dots were counted digitally by Image Pro Plus software.
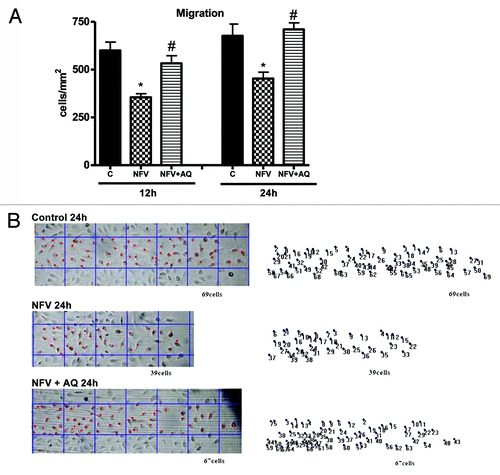
Figure 2. (A) Protective effect of supplementation with 10mM of alanyl-glutamine (AQ) on IEC-6 cell proliferation following 1h of exposure to nelfinavir (NFV) at 70μg/mL . (B) Effect of 1, 5, 10 and 50mM of AQ supplementation on IEC-6 cell proliferation, evaluated with a colorimetric assay by detecting absorbance using an ELISA microplate-reader at 450 nm. After 24 and 48 h, wells were incubated for 4 h with 10 μL of tetrazolium salt and the absorbance was measured. After 24 and 48 h, wells were incubated for 4 h with 10 μL of tetrazolium salt and the absorbance was measured. Values are expressed as mean ± standard error. *p < 0.05, compared with control group with media without glutamine, by student’s unpaired t test. #p < 0.05, compared with group with NFV, by student’s unpaired t-test.
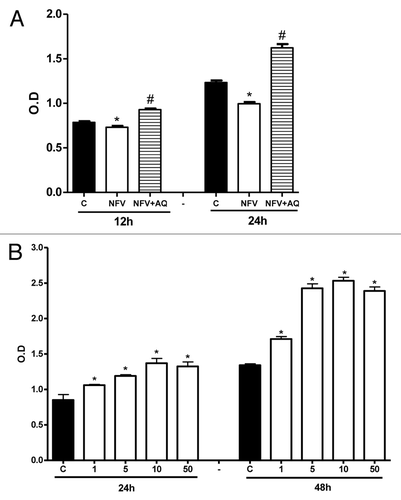
Figure 3. Flow cytometry analysis for apoptosis and necrosis rates on intestinal epithelial IEC-6 cell apoptosis and necrosis following 24 h of NFV treatment at 70 μg/mL, with or without supplementation with 10 mM of alanylglutamine (AQ). Control group (A), NFV (B), NFV+AQ (C). Lower-right quadrant represent apoptotic cells (high annexin V-FITC and low propidium iodide staining), lower-left quadrant indicate viable cells (low annexin V-FITC and propidium iodide staining), and upper-right quadrant show necrotic cells (high propidium iodide and annexin V-FITC staining). Graph (D) indicates the effect of both 1 h and 24 h exposure to NFV at 70 μg/mL and supplementation with AQ at 10 mM.
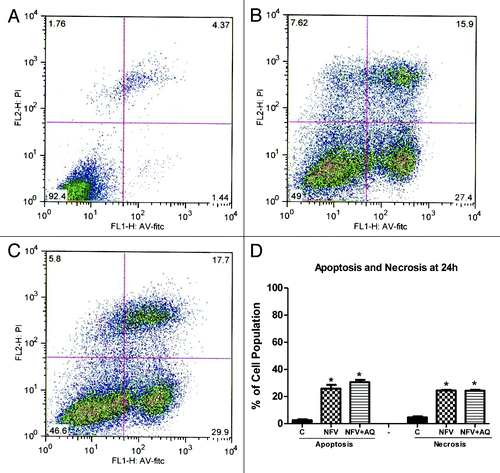
Figure 4. (A) Effect of nelfinavir at 100 mg/kg (NFV+PBS) and supplementation with 10 mM of alanyl-glutamine (NFV+AQ) compared with control (PBS), on body weight variation during 7-d treatment. For each animal (n = 6), daily body weight was measured. (B) Effect of NFV and AQ supplementation (NFV+AQ) on jejunum villi area, villli height, crypt depth and (C) morphometry after 7 d of treatment. For each animal (n = 6), 10 measurements of each small intestine segment were taken. Values are expressed as percentage of control. *p < 0.05, compared with PBS control group, by student’s unpaired t-test. #p < 0.05, compared with group with NFV, by one-way ANOVA, with Bonferroni’s post test.
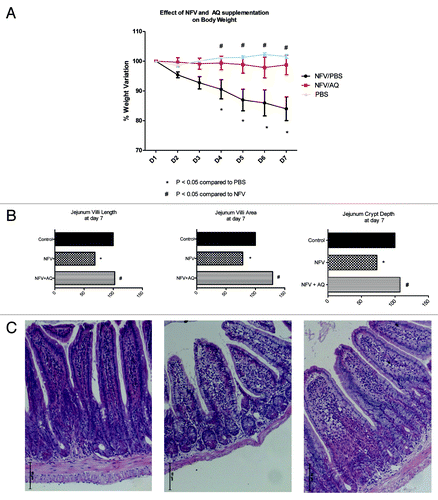
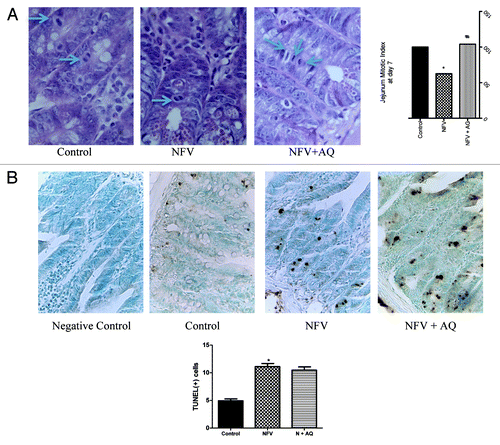
Figure 5. (A) Effect of nelfinavir at 100 mg/kg (NFV) and supplementation with 10 mM of alanyl-glutamine (NFV+AQ) on jejunum intestinal morphometry and mitotic index compared with control group (CONTROL). Values are expressed as percentage of control. (B) Effects of NFV and AQ supplementation (NFV+AQ) on cell death in the jejunum after 7 d of treatment compared with the PBS control (C). For each animal (n = 6), intestinal samples were collected and stained with TUNEL for immunohistochemistry. All slices were counterstained with methyl green, designed for nuclear counterstaining (stained light green, similar to blue color), which provide excellent contrast to brown. A strong methyl green stain, observed in the negative control slice, means that no immunostaining was detected, as expected. Negative control represents a sample of the jejunum where the antibody was replaced by 5% PBS/BSA. *p < 0.05, compared with PBS control group, by student’s unpaired t test. #p < 0.05, compared with group with NFV, by one-way ANOVA, with Bonferroni’s post-test.