Figures & data
Figure 1. Ectopic alteration of NDRG1 expression level in KYSE30 cells and its effect on cell proliferation. “NDRG1+” refers to transfectants ectopically overexpressing NDRG1, here and elsewhere. (A) Western blot analysis for NDRG1, p27Kip and p21Cip. (B) Anchorage dependent proliferation of wild type KYSE30 cells and transfectants with altered NDRG1. The MTS proliferation assay was measured at 490 nm. Each point represents the mean of four readings in one experiment. Bars, ± SD.
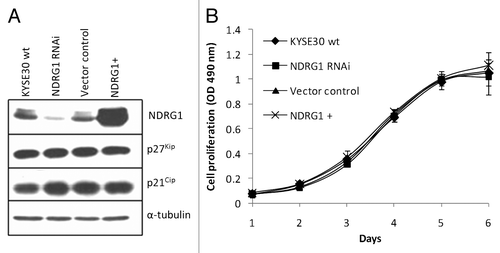
Figure 2. The effect of NDRG1 on cell migration, invasion and expression of angiogenic cytokines. (A) Representative images (100 × magnification) of the scratch/wound-healing assay. The scratched “gaps” are highlighted with black lines. (B) Transwell migration and invasion assay. Cells that migrated through plain (migration) or Matrigel coated (invasion) porous membranes were quantified 48 h after seeding. (C) Gelatin zymography to detect gelatinases MMP-2 and MMP-9 activity. An aliquot of concentrated culture media representative of the same number of KYSE30 wild type cells and transfectants was subjected to the zymography assay. One of the three independent experiments is shown. (D) Quantitative real-time RT-PCR to determine the effect of NDRG1 on the mRNA levels of MMP-2 and MMP-9. The expression has been normalized to β-actin mRNA. (E and F) The mRNA levels of VEGF-A, VEGF-C, Angiopiotein-1 (Ang-1), PDGF-A, PDGF-B, GRO-α/CXCL1, GRO-β/CXCL-2, IL-6/INF-β and IL-8/CXCL-8 were determined by quantitative real-time RT-PCR. Expression has been normalized to β-actin mRNA. Columns, mean of the normalized data from three independent experiments; Bars, ± SD; * p < 0.05 vs. wild type control.
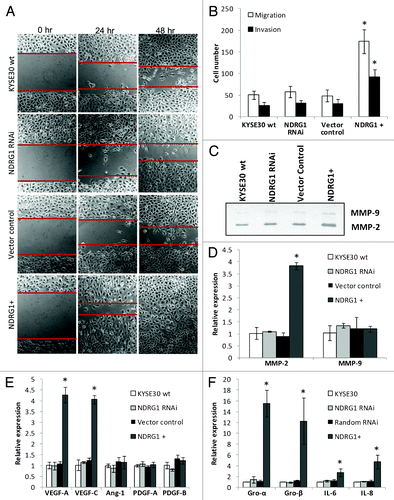
Figure 3. The effect of NDRG1 on apoptosis. (A) Subconfluent cells were challenged with indicated reagents for 24 h. Total cell lysate (including the floaters) was subjected to western blot analysis to detect the intact (p116) and cleaved (p85) forms of PARP. (B) Caspase3/7 activities were determined after 24 h treatment of indicated reagents, and were plotted as fold induction against untreated cells. Abbreviations for treatments: Doxo: 2 μM doxorubicin; Cis: 5 μg/mL cisplatin; Ni: 1 mM NiSO4; Co: 1 mM CoCl2. Columns, mean of the normalized data from three independent experiments; Bars, ± SD; * p < 0.05 vs. wild type control.
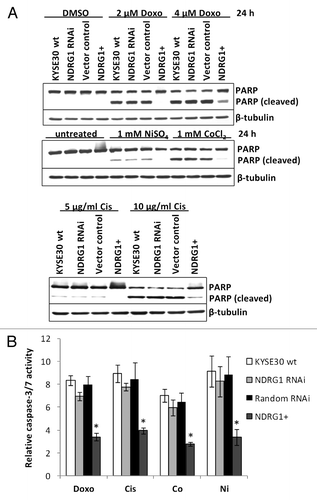
Figure 4. NDRG1 overexpression enhances p65 nuclear localization and binding to the NFκB motif. (A) Whole cell lysates, along with cytoplasmic and nuclear protein fractions from KYSE30 cells and transfectants were subjected to western blot analysis to detect levels of p65 in the nucleus. Antibodies to PARP and GAPDH were used to determine the degree of cytoplasmic and nuclear cross-contamination, respectively. (B) Confocal microscope images, taken through the plane of the nucleus, showing p65 localization in KYSE30 cells and KYSE30 NDRG1 + cells untreated, or treated with Cisplatin (C), ChIP assays to demonstrate the p65/Rel-A dependent upregulation of GRO-β/CXCL-2 in NDRG1 overexpressing KYSE30 cells. ChIP assay was performed with p65/Rel-A antibody and null antibody as control in parental and NDRG1 overexpressing KYSE30 cells, respectively. Quantitative PCR was performed with specific primers flanking the p65/Rel-A consensus site of the GRO-β/CXCL-2 promoter. Both the PCR product (370 bp) and the qPCR result of the fold change corrected with non-specific interaction, detected in the no-antibody control ChIP, are shown; * p < 0.05 vs. wild type control.
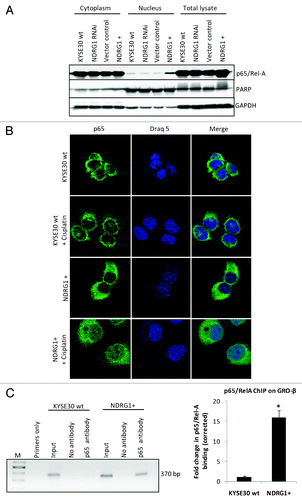
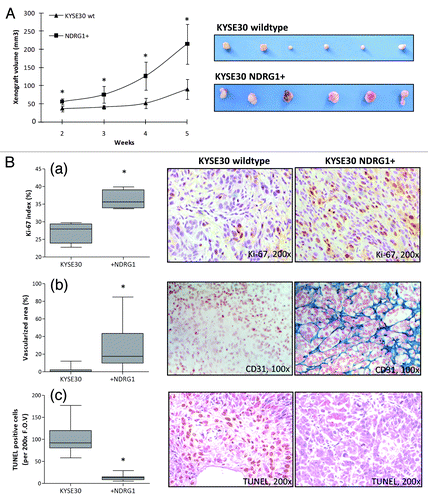
Figure 5. Nude mice xenograft model to explore the in situ growth of KYSE30 wild type cells (KYSE30 wt) and transfectants with constitutive NDRG1 overexpression (NDRG1+) (n = 6 in each group). (A) 5 × 106 cells were inoculated subcutaneously into the dorsal flank. Photographs of the dissected xenografts with the same magnification scale are shown on the right. Dots, mean of the xenograft volume of all the six mice in the group; Bars, ± SD; significant difference in tumor volume was observed from week 2, * p < 0.05. (B) Analysis of proliferative activity, apoptosis and vascularization in histological sections of the xenografts derived from KYSE30 wt and NDRG1+ cells, with the quantitative results shown on the left and representative images (× 100) shown on the right. Columns, mean data of the sections from the six mice in the group; Bars, ± SD; (*) p < 0.05. (a) Proliferative activity was assessed by Ki-67 staining; (b) vascularization was determined by staining for CD31 and (c) apoptosis was measured by terminal TUNEL assay, as described in the methods section.