Figures & data
Figure 1. Schematic representation of the glucose metabolism in cancer cells. Cancer cells increase the uptake and metabolism of glucose by regulating key transporters and enzymes (shown in red font) involved in glycolytic pathways. Key oncogenic pathways are shown in green and key tumor suppressor pathways are shown in purple. The oxidative branch of PPP is required in nucleotide synthesis and dihydroxyacetone phosphate pathway is critical for lipid synthesis.
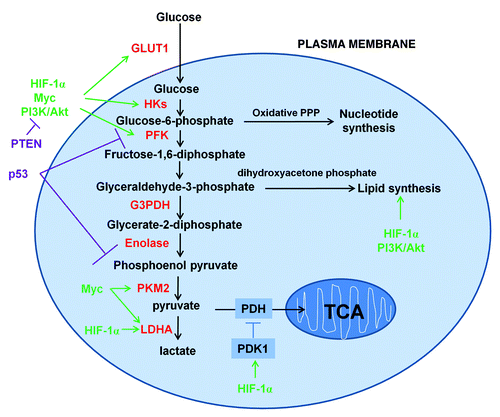
Table 1. Summary table of selective potential drugs/compounds targeting cancer metabolism pathways and mode of action
Figure 2. Mitochondrial oxidative phosphorylation in cancer cells. Some energy-rich metabolites (L-lactate, ketones and fatty acids shown in yellow) derived from the tumor stroma can be transferred to the adjacent cancer cells and used for energy production via mitochondrial oxidative phosphorylation.
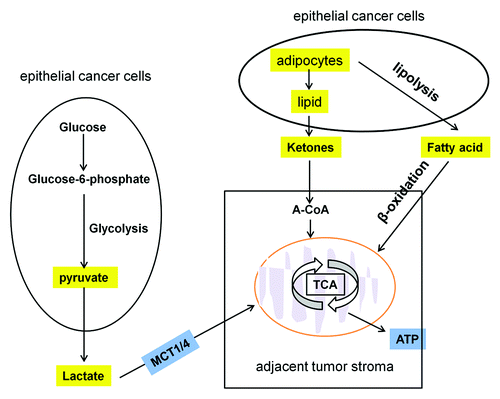
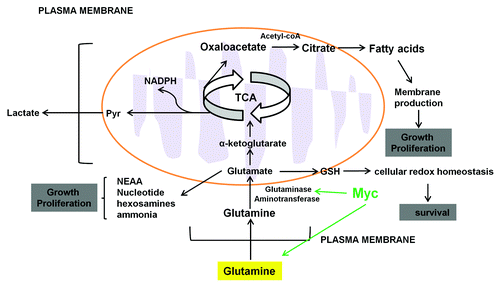
Figure 3. Glutamine metabolism in cancer cells. High throughput glutamine uptake feeds cancer cell growth and proliferation with a large pool of carbon and nitrogen for the biosynthesis of the nonessential amino acids and fatty acids for membrane production. Carbon precursors derived from glutaminolysis (oxaloacetate and glutamate) serve as the carbon substrate for amino acid biosynthesis and lactate. Glutamate donates its amine group to these carbon substrates to produce non-essential amino acids (NEAA), nucleotide and α-ketoglutarate. Glutamine can be converted directly into GSH which has antioxidant role and plays a key role in controlling cellular redox homeostasis.