Figures & data
Figure 1. Macitentan in vitro cytotoxicity with and without chemotherapy. (A) qRT-PCR demonstrating the expression of ETRA and ETRB by human (1) and mouse (2) ovarian cancer and endothelial cell lines. Human ovarian cancer cell lines include A2008, A2780 and Ovcar5. Human dermal microvascular endothelial cells (HDMEC) are used as endothelial source. The murine ID8 ovarian cancer cell line and MS1 and bEnd.3 (brain endothelial cells) are used as a source of endothelial cells. (B) Viable cell percentage (relative to DMSO controls) of tumors cells (1) and endothelial cells (2) treated with increasing does of macitentan. Macitentan demonstrates activity (1C50 ~1uM) vs. both cell lines (A2008 and A2780) which demonstrate ETRA expression, but not against OVCAR5 cells which do not express ETRA . Macitentan demonstrates activity against both human and murine endothelial cells. ET-1 and DMSO treatment are shown as controls. (C) Macitentan cytotoxicity in A2008 cells in combination with Cisplatin demonstrates additive activity.
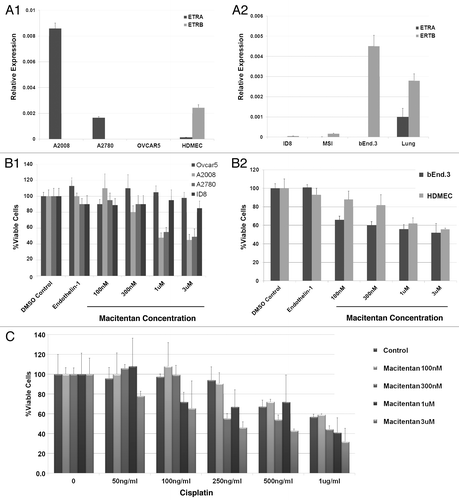
Figure 2. Macitentan treatment of ID8 tumors with and without vaccine therapy. (A) ID8 tumors injected in mice treated with macitentan alone, ID8 cell vaccination alone or combination of macitentan and vaccine. Tumor volume over 56 d demonstrates modest activity of the tumor vaccine but no additional activity with the addition of macitentan. (B) ID8 tumors in mice treated with ID8 cell vaccination alone, vaccine + BQ788 (ETRB antagonist) or vaccine + BQ788 (ETRB antagonist) + BQ123 (ETRA antagonist). Tumor volume over 56 d was measured demonstrating additive activity of vaccine+BQ788 which is inhibited in the presence of ETRA antagonism with BQ123. (C) Immunohistochemical CD8 labeling of ID8 tumors treated in B demonstrating increased CD8 T cell infiltration in tumors treated with vaccine and BQ788. D. Levels of ICAM-1 mRNA in HDMEC cells after 24 h of treatment with ET-1 (necessary for increased ICAM-1 expression) and BQ123, BQ788, BQ123 + BQ788 or macitentan.
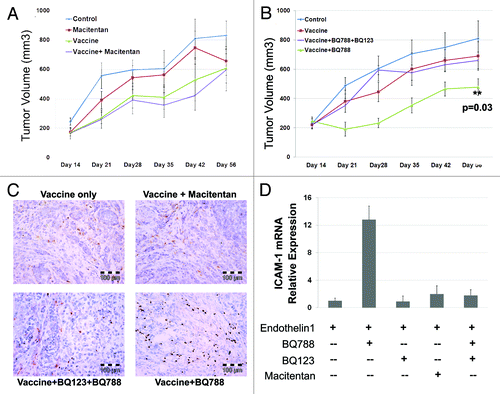
Figure 3. Macitentan treatment of ovarian cancer human tumor xenografts. (A) (1) A2008 xenografts and (2) A2780 xenografts treated with macitentan daily vs. placebo demonstrating increased survival for A2780 tumors treated with macitentan. (B) (1) A2008 and (2) A2780 xenografts treated with cisplatin with and without macitentan. Once again the addition of macitentan improved survival in the A2780 tumors only.
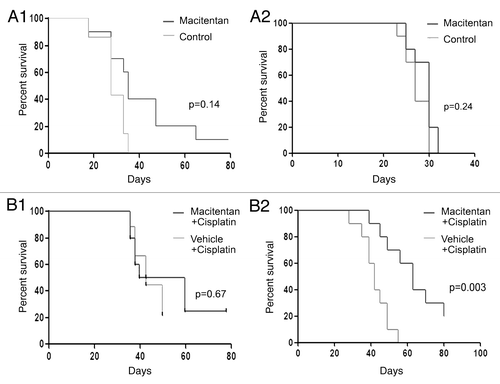
Figure 4. ETRA is preferentially expressed in CD133+ ovarian cancer stem cells. (A) qRT-PCR demonstrating a lack of differential expression of ETRA in ADLH (+) and ALDH (-) cell populations of SKOV3 and A2008 cell lines (which do not express CD133). (B) qRT-PCR demonstrating preferential expression of ETRA in CD133+ cells from A2780 and PEO1 cell lines. (C) qRT-PCR demonstrating preferential expression of ETRA in ALDH+CD133- ALDH-CD133+ and ADLH+CD133+ primary human ovarian cancer stem cells vs. ALDH-CD133- progenitor cells.
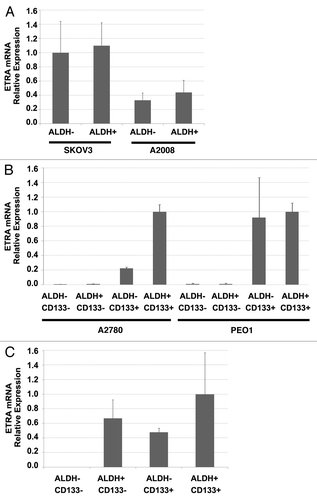
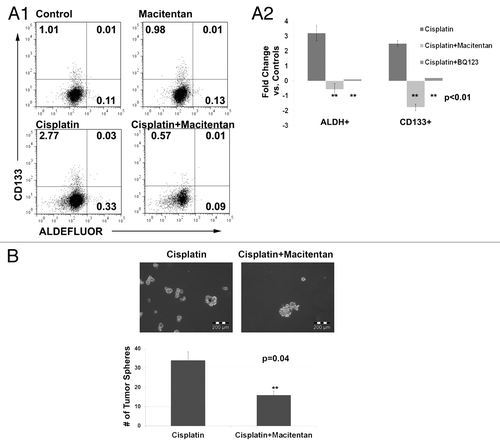
Figure 5. (A) FACS analysis (1) and average fold change (2) of A2780 cells treated with cisplatin, macitentan, or cisplatin + macitentan. Cell percentages are indicated in the respective quadrants. (B) Tumor spheres formed from 5,000 ascites cells collected from a platinum refractory ovarian cancer patient treated with cisplatin or cisplatin + macitentan.