Figures & data
Figure 1. RNAi-mediated stable knockdown of CDH17 in MKN-45 cells. (A) The plasmids of pRI-CMV/eGFP-CDH17-miR-1, -2, -3 and -neg detected by DNA sequencing were consistent with the designed miRNA insert fragments. (B) High expression of GFP was observed in lentiviral vector transfected cells, as opposed to the negative expression of it in MKN-45 cells receiving no treatment (100μm). (C) 94–98% of transfected cells are GFP positive, determined by flow cytometry analysis. (D) Real time RT-PCR indicated that the miR-CDH17(2) was the optimal sequence, with 80% interference efficacy of CDH17, which was confirmed by (E) western blot analysis.
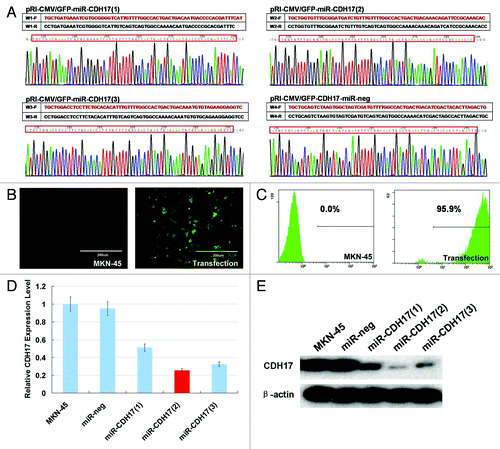
Figure 2. Inhibition of CDH17 repressed the malignant potency of MKN-45 cells and alleviated the activation of NFκB signaling pathway. (A) Immunofluorescence staining demonstrated that CDH17 was significantly downregulated in miR-CDH17 cells, compared with the high expression in both MKN-45 and miR-neg cells (100μm). (B) Proliferation assay. (C) Adhesion assay. (D) Cell cycle analysis. (E) Invasion assay (100μm). (F) In western blot analysis, there was less expression of CDH17, nuclear p65, MMP-9 and VEGF-C, but more expression of IκB-α in miR-CDH17 group. There was no difference in total p65 among each group.
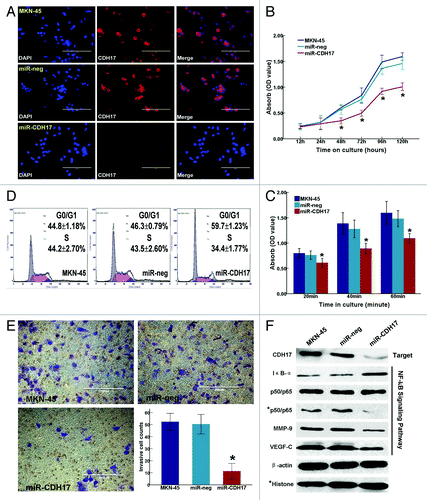
Figure 3. Knockdown of CDH17 inhibited tumor growth and the expressions of NF-κB, MMP-9 and VEGF in tumor tissue. (A) Upon the termination, masses with fluorescence were larger in mock and miR-neg groups than those in miR-CDH17 group, which was coincident with the gross specimen (B, C). (D) The line chart illustrated that the tumor volume of miR-CDH17 was smaller than the other two groups consecutively, p < 0.05. (E) Expressions of p65 (NFκB), VEGF-C and MMP-9 were all suppressed in tumor tissue of miR-CDH17 nude mice (100μm). There was no significant difference between those in mock group and miR-neg group.
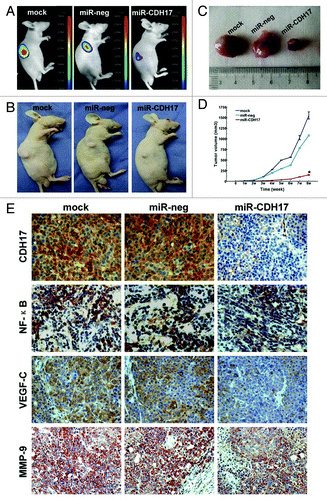
Figure 4. Knockdown of CDH17 expression inhibited lymph node metastasis in vivo. (A) The morphological characteristics of claw pads in each group were similar at the beginning, and then tumor nodules of the controls grew much larger than those of the miR-CDH17 group. (B) Two mice in both mock group and miR-neg group developed metastasis in the inguinal region, as shown by the presence of GFP-positive tumor cells. However, there was no fluorescent lesion detected in the mice of miR-CDH17 group, which was confirmed by (C) the anatomical gross specimens. (D) HE and immunohistochemisty of AE1/AE3 staining showed that the enlarged lymph nodes in mock and miR-neg group were occupied by tumor cells, as opposed to the normal lymph node in miR-CDH17 group. Additionally, CDH17 in lymphatic lesions expressed as high as it in primary ones (100 μm).
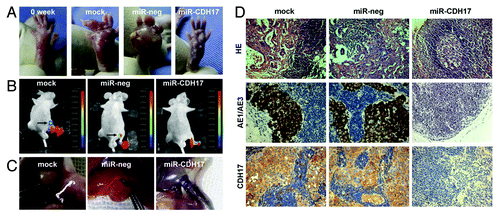
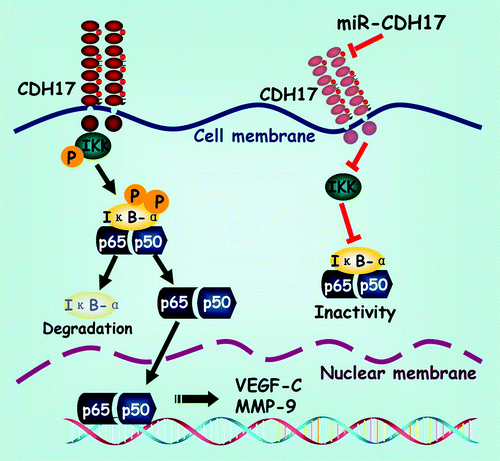
Figure 5. Schematic exhibition of the proposed signaling pathway modulated by CDH17 in gastric cancer cells. Coupling CDH17 activates the IKK complex, which subsequently phosphorylates the IκB-α. Then the phosphorylated IκB-α undergoes proteasome-dependent decomposition, which releases the heterodimers of p65/p50 into cytoplasm and transferred into the nucleus. Finally, the p65 binds to its responsive gene and promotes the transcription of downstream proteins including VEGF-C and MMP-9. On the other hand, RNAi mediated inhibition of CDH17 attenuates the activation of NFκB in gastric cancer cells, leading to a concomitant reduction in downstream proteins.