Figures & data
Figure 1. RON is highly expressed in Burkitt’s lymphoma (BL) and Hodgkin’s lymphoma (HL) tissues and cells. (A) Western blots show RON expression in various human leukemia/lymphoma cell lines. Cellular protein samples (50 µg) were subjected to western blot analysis using rabbit immunoglobulin G to the RON C-terminus (R5029). 3T3-RON cells were used as positive controls. Actin served as a loading control. (B) RON expression in clinical samples was detected by western blot analysis. Lane 7, Burkitt’s lymphoma specimen; lanes 10 and 17, 3T3-RON cells; lanes 11 and 16, Hodgkin’s lymphoma specimens. (C) RON immunostaining and EBV in situ hybridization (ISH) in BL and HL lymphoma tissues. Upper panel, cases in which routine histological diagnosis was determined by H&E staining; middle panel, representative RON immunohistochemical (IHC) staining of BL and HL tissue samples; bottom panel, strong nuclear EBV ISH in BL and HL samples by EBER. Images were obtained using a BK40 Olympus microscope (magnification, ×40).
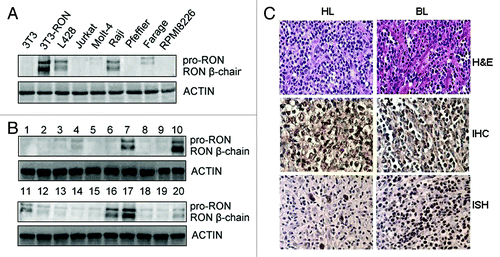
Table 1. RON expression in various types of lymphoma tissue, as assessed by immunohistochemical staining
Table 2. Correlation between RON overexpression (percentage) and EBV infection cases in BL and HL
Figure 2. Effect of Zt/f2 treatment on Raji cell proliferation and survival in vitro. (A) Raji cell viability following Zt/f2 treatment was determined using an MTT assay. Exponentially growing cells were seeded into 96-well plates and triplicate samples were treated with macrophage-stimulating protein (MSP; 2 nM) in the presence or absence of Zt/f2 (2 nM) or control mouse immunoglobulin G (2 nM). Cell proliferation was determined using an MTT assay at different time points. Jurkat cells were used as negative controls. (B) Effect of Zt/f2 on Raji cell colony formation in methylcellulose. Cells (1 × 103/well) were seeded onto 0.8% methylcellulose and 20% FBS/RPMI-1640 in triplicate in 24-well plates and then MSP (2 nM), Zt/f2 (2 nM) or both were added. Jurkat cells were used as negative controls. Colony (Col.) numbers (for colonies containing ≥30 cells) were counted after 10 d; #, *, p < 0.05 vs. control group at 72 h.
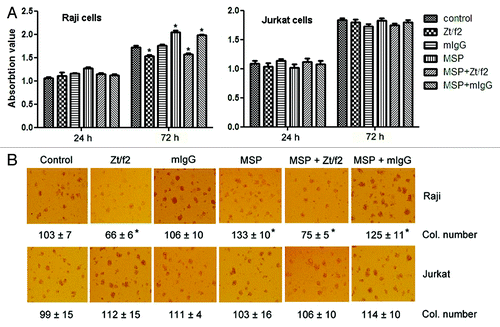
Figure 3. Zt/f2 treatment induces apoptosis and cell cycle arrest. Raji cells were treated with macrophage-stimulating protein (MSP; 2 nM) in the presence or absence of Zt/f2 (2 nM) or control mouse immunoglobulin G (2 nM) for 72 h. (A) The proportion of early apoptotic cells in each quadrant is indicated. Data show that Zt/f2 treatment alone moderately increases the percentage of early apoptotic cells. A slight increase in the proportion of apoptotic cells is observed following combined MSP and Zt/f2 treatment. (B) Zt/f2 treatment induces G1-phase cell cycle arrest in Raji cells. (C) Zt/f2 regulates the expression of apoptosis-related proteins in Raji cells. Treatment of Raji cells with Zt/f2 downregulates anti-apoptotic proteins (Mcl-1 and XIAP) and upregulates pro-apoptotic proteins (caspases 3 and 9, and poly [ADP-ribose] polymerase [PARP]). (D) Western blot analysis shows that expression of cyclins and cyclin-dependent kinases (cyclin D1, CDK4 and CDK6) are significantly reduced and p27 expression is significantly increased in Zt/f2-treated Raji cells.
![Figure 3. Zt/f2 treatment induces apoptosis and cell cycle arrest. Raji cells were treated with macrophage-stimulating protein (MSP; 2 nM) in the presence or absence of Zt/f2 (2 nM) or control mouse immunoglobulin G (2 nM) for 72 h. (A) The proportion of early apoptotic cells in each quadrant is indicated. Data show that Zt/f2 treatment alone moderately increases the percentage of early apoptotic cells. A slight increase in the proportion of apoptotic cells is observed following combined MSP and Zt/f2 treatment. (B) Zt/f2 treatment induces G1-phase cell cycle arrest in Raji cells. (C) Zt/f2 regulates the expression of apoptosis-related proteins in Raji cells. Treatment of Raji cells with Zt/f2 downregulates anti-apoptotic proteins (Mcl-1 and XIAP) and upregulates pro-apoptotic proteins (caspases 3 and 9, and poly [ADP-ribose] polymerase [PARP]). (D) Western blot analysis shows that expression of cyclins and cyclin-dependent kinases (cyclin D1, CDK4 and CDK6) are significantly reduced and p27 expression is significantly increased in Zt/f2-treated Raji cells.](/cms/asset/30ca95b9-18fc-47ca-92e2-14e453cfbe8b/kcbt_a_10923718_f0003.gif)
Figure 4. Zt/f2 inhibits RON phosphorylation and downstream signaling. (A, B) Macrophage-stimulating protein (MSP)-induces phosphorylation of RON and downstream signaling proteins in Raji cells. Cells were stimulated with MSP (2 nM) for different periods of time. Phosphorylation of RON at tyrosine residues was detected by western blotting with the mAb PY-100, which is specific for phospho-tyrosine residues. β-Catenin and phosphorylated/total Akt, ERK1/2, p38 and p65 were also measured by western blotting using specific antibodies. Actin was used as a loading control. (C, D) RON inhibition by Zt/f2 inhibits phosphorylation of Akt, Erk1/2, P38 and P65 in Raji cells. Cells were treated with 2 nM MSP, 2 nM Zt/f2 or both for 60 min followed by western blot analysis using antibodies specific to phosphorylated and total RON, Akt, Erk1/2, P38, P65 and β-catenin.
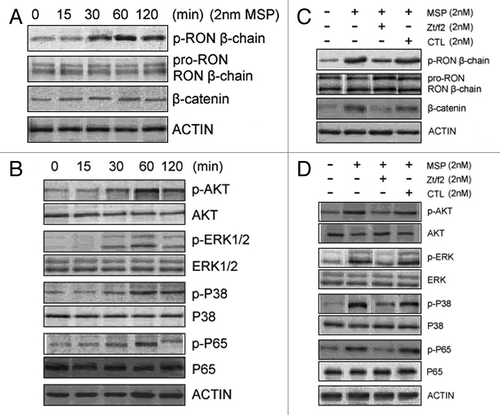
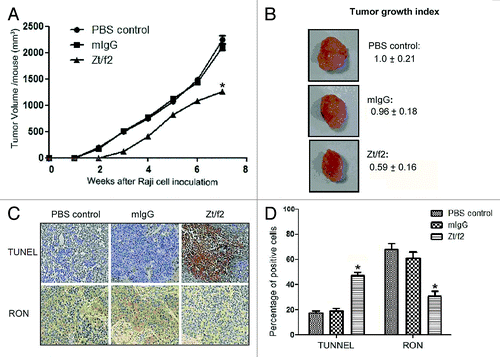
Figure 5. Zt/f2 treatment inhibits Raji cell xenograft growth. (A) Zt/f2 treatment significantly suppresses the growth of Raji cell xenografts in athymic nude mice. Tumor-bearing mice were randomized into different groups (three mice per group). Animals were treated with Zt/f2, PBS or control mouse IgG at the doses and schedule defined in the Materials and Methods section. For each treatment group, the tumor volume was measured weekly. Tumor xenograft tissues were harvested after 7 weeks. (B) The tumor growth index in control mice was set to 1.00 and used to make comparisons among groups. (C, D) Immunohistochemical detection of TUNEL-positive and RON+ cells in tumor xenograft tissues. The percentage of positively stained cells in the tissues of each group is shown. TUNEL-positive and RON+ cells were counted in four or five different fields, the data are summarized as the mean of percentage positive cells in three xenograft samples. The proportion of apoptotic cells was significantly increased and there was a clear downregulation of RON in Zt/f2-treated samples. *, p < 0.05 vs. PBS control group.