Figures & data
Figure 1. ATO has a weak apoptosis effect in renal cell carcinoma cells and upregulates galectin-3 (Gla-3) protein expression. (A) ATO treatment (2.5 μM) did not significantly induce apoptosis in RCC cells, especially in Caki-1 and 786-0 cells. Cells were treated with ATO followed by staining with PI and Annexin V as described in Materials and Methods. Apoptotic analysis was applied to cells before (top panel) and after (bottom panel) ATO treatment using flow cytometer, and the percentage of each population is labeled in the figure. (B) ATO treatment (2.5 μM) increased endogenous galectin-3 expression in 786-0, ACHN, Caki-1 and Caki-2 cells. Cells were treated with or without ATO as described in Materials and Methods. Anti-Gal-3 antibody (1:1000) was used to detect endogenous Gal-3 proteins. GAPDH was used as loading control. Quantifications of respective Gal-3 levels are presented based on at least three-time repeats. *P < 0.05, **P < 0.01.
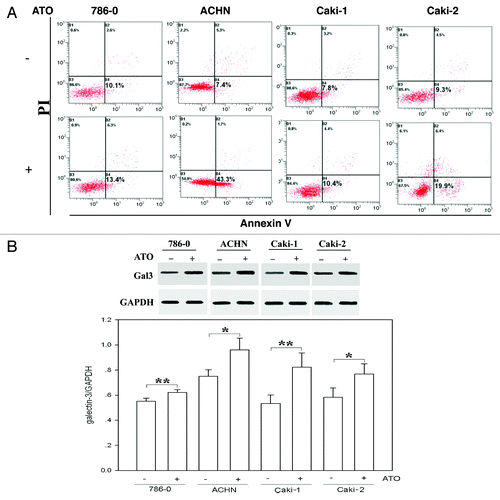
Figure 2. ATO induces the translocation of Gal-3 from the nucleus to the cytoplasm. (A) Gal-3 distribution before and after ATO treatment. The red signal shows Gal-3, and the blue one is nuclei. The staining results in 786-0, ACHN, Caki-1, and Caki-2 cells are presented from top to bottom, respectively. Gal-3 transferred from the nucleus to the cytoplasm in Caki-1 and 786-0 cells. (B and C) Western blotting was used to confirm the translocation of Gal-3. The anti-Gal-3 antibodies were used to detect Gal-3 in the nucleus and cytoplasm. Quantifications of respective Gal-3 levels are presented based on at least three-time repeats (*P < 0.05).
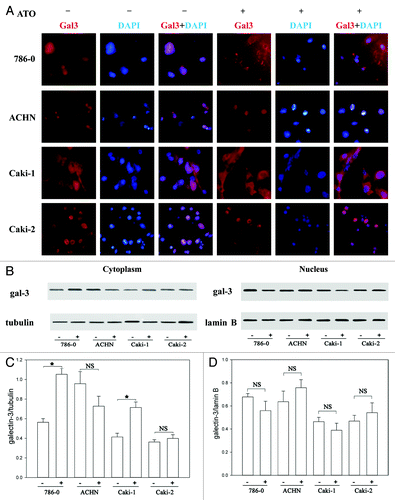
Figure 3. Synexin is co-translocated with Gal-3 in RCC cells. (A) The total protein levels of synexin were the same before and after ATO treatment in all RCC cells tested. (B) ATO triggered the translocation of synexin from the nucleus to the cytoplasm in Caki-1 and 786-0 cells. The protein levels of synexin in the nucleus and cytoplasm were determined using western blotting. (C) Immunofluerescence experiment confirmed that Gal-3 (red) was co-translocated with synexin (green) in RCC cells.
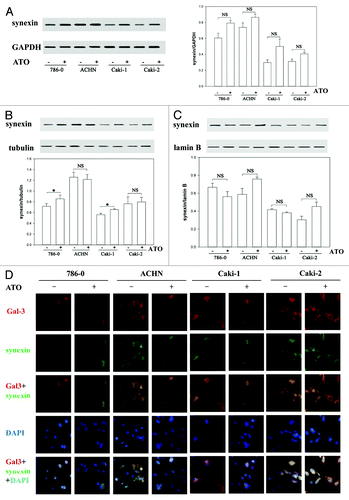
Figure 4. The sensitivity of Caki-1 cells to ATO-induced apoptosis is increased by Gal-3 knockdown. (A) The protein level of Gal-3 was reduced after shRNA treatment. Four independent shRNAs against Gal-3 were used to construct stable cell lines. (B) The apoptosis of Caki-1 cells was enhanced by Gal-3 knockdown, and ATO treatment further increased apoptotic effect. Cells were labeled with Annexin V (x-axis) and PI (y-axis), and apoptosis was analyzed using flow cytometer. (C) Cell viability was reduced by Gal-3 knockdown combined with ATO treatment.
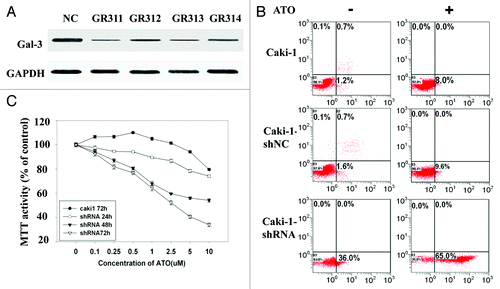
Figure 5. Gal-3 knockdown decreases mitochondrial membrane potential and increases mitochondria-related apoptosis after ATO treatment. (A) Gal-3-knockdown Caki-1 cells showed reduced mitochondrial membrane potential, and the reduction was further enhanced by ATO treatment. JC-1 mitochondrial membrane potential assay was performed as described in methods. (B) Cytochrome c was released in response to Gal-3 knockdown and ATO treatment. Anti-cytochrome c antibodies were used to detect cytochrome c in the cytoplasm. (C) Caspase-3 activity was increased and (D) cell viability was reduced after Gal-3 knockdown and ATO treatment (P < 0.05).
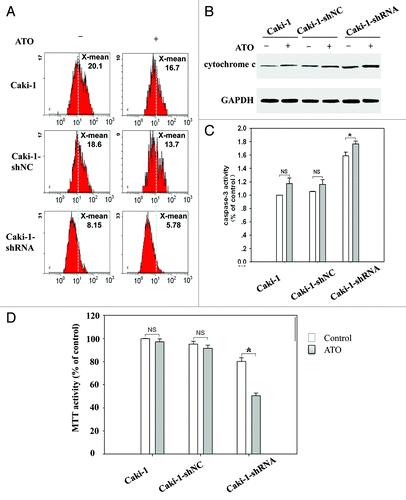
Figure 6. Gal-3 knockdown increases the apoptotic effect of ATO in RCC cells. (A) Gal-3 protected Bcl-2 from ATO induced reduction. (B) Gal-3 did not affect Bcl-xl levels before and after ATO treatment. (C) The Galecin-3 inhibitor, MCP, facilitated ATO to induce aggressive apoptosis in RCC cells, including 786-0, ACHN, Caki-1, and Caki-2.
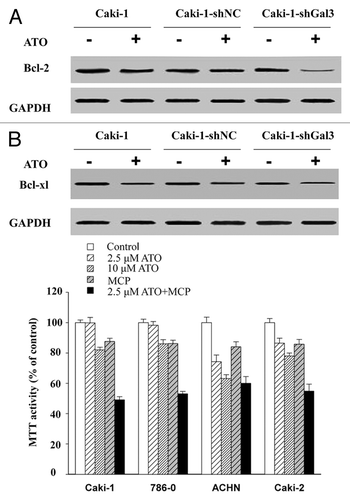
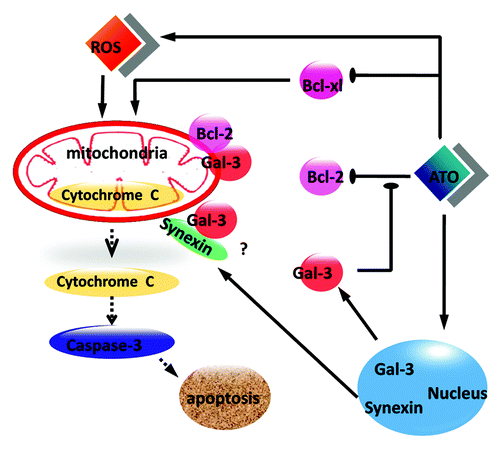
Figure 7. A scheme illustrates the anti-apoptotic role of Gal-3 in ATO-treated RCC cells. ATO increases the protein level of Gal-3 and triggers the co-translocation of Gal-3 from the nucleus to the cytoplasm with synexin. Cytoplasmic Gal-3 blocks the Bcl-2 reduction medicated by ATO and further protects mitochondrial membrane potential so that the cytochrome c release is decreased.