Figures & data
Figure 1. Low and high dose lapatinib increase obatoclax toxicity. BT474 and GBM12 cells in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM or 100 nM), obatoclax (GX, 50 nM) or the drug combination. Cells were isolated as indicated 12, 24, and 48 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in WT cells ##P > 0.05 compared with vehicle treated cells; *P < 0.05 greater than vehicle control.
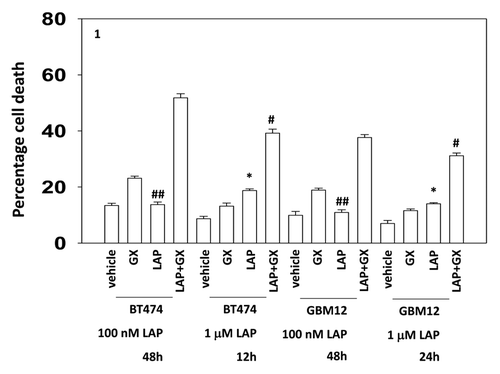
Figure 2A–D. Development of anoikis resistance in breast cancer cells. (A) BT474 cells (wild type; anoikis-resistant) were plated and 24 h later cells were isolated for immunoblotting against the indicated proteins and phospho-proteins (n = 3). (B) MCF7 cells (wild type; anoikis-resistant) were plated and 24 h later cells were isolated for immunoblotting against the indicated proteins and phospho-proteins (n = 3). (C) SKBR3 cells (wild type; anoikis-resistant) were plated and 24 h later cells were isolated for immunoblotting against the indicated proteins and phospho-proteins (n = 3). (D) BT474 cells (wild type; anoikis-resistant) were assessed for the expression of ERBB1 and ERBB2, and the impact of lapatinib (1 μM) on the phosphorylation of these proteins (n = 3); BT474 (wild type; anoikis-resistant) cells were fixed but not permeabilized and the levels of cell surface ERBB1 and ERBB2 determined.
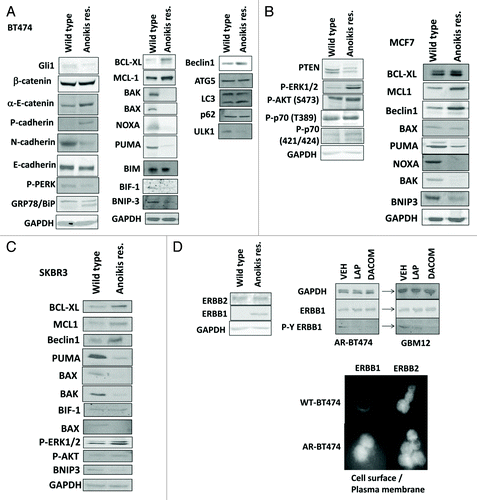
Figure 2E–I.(E) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in WT cells. (F) MCF7 cells (WT, wild type; AR, anoikis-resistant,) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Cells were isolated 24 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in WT cells. (G) SKBR3 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in WT cells. (H) BT474 cells (WT, wild type; AR, anoikis-resistant) in sextuplicate were plated as single cells in soft agar, thus growing in 3 dimensions. Twelve hours after plating cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. After 12 h the media was changed and replaced with drug free media. Colonies were permitted to form for the following 10 d, before fixing, staining and counting (± SEM, n = 3) #P < 0.05 less than corresponding value in vehicle-treated cells; ##P < 0.05 less than value in lapatinib treated cells. (I) GBM6 and GBM12 cells (WT, wild type; AR, partially anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Cells were isolated 24 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in WT cells.
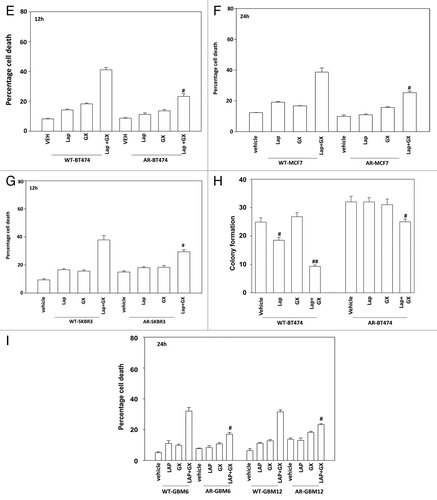
Figure 5. HDAC inhibitors restore expression of toxic BH3 domain proteins in anoikis-resistant breast cancer cells. (A) BT474 (WT, wild type; AR, anoikis-resistant) cells were treated for 24 h with vehicle or with either sodium valproate (750 μM) or vorinostat (750 nM). Cells were isolated and immunoblotting performed to determine expression of PUMA, BAX, BAX, and NOXA (n = 3). (B) BT474 and SKBR3 (WT, wild type; AR, anoikis-resistant) cells were treated for 36 h with vehicle or with either sodium valproate (750 μM) or vorinostat (750 nM). Cells were isolated and viability determined by trypan blue (± SEM, n = 3). (C) BT474 (WT, wild type; AR, anoikis-resistant; LAP-R, lapatinib-resistant) cells were treated for 24 h with vehicle or with either sodium valproate (750 μM) or vorinostat (750 nM). Cells were washed and the media was replaced with drug free media. Cells were then treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in vehicle-treated cells. (D) GBM6 and GBM12 cells (WT, wild type; STEM, stem cells) cells were treated for 24 h with vehicle or with sodium valproate (750 μM). Cells were washed and the media was replaced with drug-free media. Cells were then treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 24 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in vehicle-treated cells. (E) BT474 (WT, wild type; AR, anoikis-resistant) cells were transfected with scrambled siRNA (siSCR, 20 nM) or siRNA molecules to knock down BAK, BAK, NOXA, and PUMA, as indicated. cells were treated for 24 h with vehicle or with sodium valproate (750 μM). Cells were washed and the media was replaced with drug free media. Twenty-four hours after transfection cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in valproate treated siSCR cells.
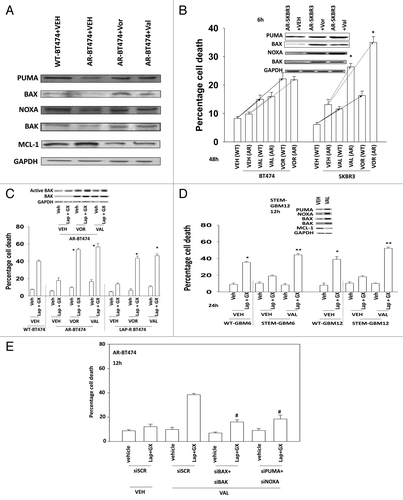
Figure 3. Lapatinib-resistant BT474 cells overexpress MCL-1 and BCL-XL and have reduced expression of toxic BH3 domain proteins. (A) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), dacomitinib (dacom., 100 nM), obatoclax (GX, 50 nM), or the drug combination. Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than others values in (dacom. + GX) cells; **P < 0.05 greater than VEH cells. (B) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), afatinib (AFA, 100 nM), obatoclax (GX, 50 nM) or the drug combination. Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than others values in (AFA. + GX) cells; **P < 0.05 greater than VEH cells. (C) BT474 cells (WT, wild type; AR, anoikis-resistant) were plated in a Millipore Millicell. The migration of cells was determined after 12 h (± SEM, n = 3) *P < 0.05 greater than corresponding value in WT cells.
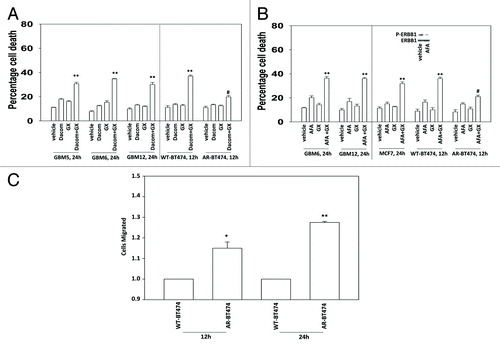
Figure 4. Regulation of lapatinib + obatoclax lethality by toxic BH3 domain proteins. (A) BT474 (wild type) cells were transfected with scrambled siRNA (siSCR, 20 nM) or siRNA molecules to knock down BAK, BAK, NOXA, and PUMA, as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in siSCR cells; ##P < 0.05 less than corresponding single knock down cells. (B) BT474 (WT, wild type; AR, anoikis-resistant) cells were transfected with empty vector plasmid (CMV) or plasmids to express BAX, BAK, NOXA, and PUMA as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in CMV cells; **P < 0.05 greater than corresponding single expression cells. (C) BT474 (AR, anoikis-resistant) cells were transfected with scrambled siRNA (siSCR, 20 nM) or siRNA molecules to knock down MCL-1, c-FLIP-s, and BCL-XL, as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in siSCR cells; %P < 0.05 less than corresponding value in siMCL-1 cells. (D) SKBR3 (AR, anoikis-resistant) cells were transfected with scrambled siRNA (siSCR, 20 nM) or siRNA molecules to knock down MCL-1 and BCL-XL, as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in siSCR cells; %P < 0.05 less than corresponding value in siMCL-1 cells. (E) BT474 (AR, anoikis-resistant) cells were infected with empty vector adenovirus (CMV) or with adenoviruses to express dominant negative MEK1 or dominant negative AKT, as indicated. Thirty-six hours after infection cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in CMV cells.
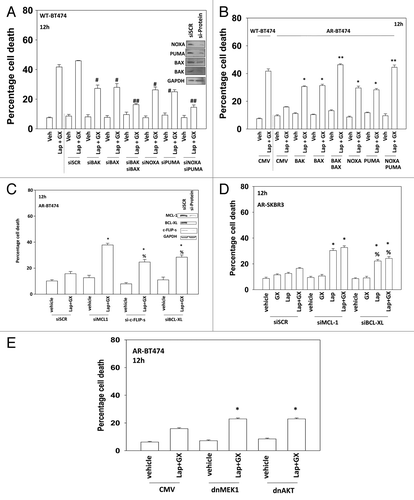
Figure 6. CDK inhibitors reduce expression of protective BCL-XL and MCL-1 proteins in anoikis-resistant breast cancer cells. (A) MCF7 and BT474 (WT, wild type; AR, anoikis-resistant) cells were treated with vehicle or with dinaciclib (10 nM), afatinib (100 nM), lapatinib (1 μM), or the drugs in combination as indicated. Cells were isolated after 24 h and viability determined by trypan blue (± SEM, n = 3). *P < 0.05 greater than corresponding value in vehicle-treated cells; #P < 0.05 less than corresponding value in wild-type treated cells. Upper inset panel: the expression of MCL-1 12 h after exposure to the indicated drugs. (B) BT474 (AR, anoikis-resistant) cells were treated for 36 h with vehicle or with either sodium valproate (750 μM) or vorinostat (750 nM). Cells were then treated with vehicle or with dinaciclib (10 nM), afatinib (100 nM), lapatinib (1 μM), or the drugs in combination as indicated. Cells were isolated after 24 h and viability determined by trypan blue (± SEM, n = 3). *P < 0.05 greater than corresponding value in vehicle-treated cells within own group; **P < 0.05 greater than corresponding value in vehicle-treated cells within whole study. (C) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. Thirty minutes after drug treatment cells were mock exposed or irradiated (4 Gy). Cells were isolated 6 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in lapatinib alone cells; **P < 0.05 greater than corresponding unirradiated cells; %P < 0.05 less than corresponding value in WT cells. (D) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. As indicated cells were treated with vehicle control or with doxorubicin (0.75 μM). Cells were isolated 6 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in lapatinib alone cells; **P < 0.05 greater than corresponding non-DOX treated cells; %P < 0.05 less than corresponding value in WT cells. (E) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated for 24 h with vehicle or with sodium valproate (750 μM). Cells were washed and the media was replaced with drug free media. Cells were then treated with vehicle (VEH, DMSO) or with [lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM)] and with as indicated doxorubicin (0.75 μM) or irradiated (n.b. 2 Gy). Cells were isolated 24 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 difference between value and LAP+GX greater than corresponding value in vehicle only-treated AR-BT474 cells.
![Figure 6. CDK inhibitors reduce expression of protective BCL-XL and MCL-1 proteins in anoikis-resistant breast cancer cells. (A) MCF7 and BT474 (WT, wild type; AR, anoikis-resistant) cells were treated with vehicle or with dinaciclib (10 nM), afatinib (100 nM), lapatinib (1 μM), or the drugs in combination as indicated. Cells were isolated after 24 h and viability determined by trypan blue (± SEM, n = 3). *P < 0.05 greater than corresponding value in vehicle-treated cells; #P < 0.05 less than corresponding value in wild-type treated cells. Upper inset panel: the expression of MCL-1 12 h after exposure to the indicated drugs. (B) BT474 (AR, anoikis-resistant) cells were treated for 36 h with vehicle or with either sodium valproate (750 μM) or vorinostat (750 nM). Cells were then treated with vehicle or with dinaciclib (10 nM), afatinib (100 nM), lapatinib (1 μM), or the drugs in combination as indicated. Cells were isolated after 24 h and viability determined by trypan blue (± SEM, n = 3). *P < 0.05 greater than corresponding value in vehicle-treated cells within own group; **P < 0.05 greater than corresponding value in vehicle-treated cells within whole study. (C) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. Thirty minutes after drug treatment cells were mock exposed or irradiated (4 Gy). Cells were isolated 6 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in lapatinib alone cells; **P < 0.05 greater than corresponding unirradiated cells; %P < 0.05 less than corresponding value in WT cells. (D) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. As indicated cells were treated with vehicle control or with doxorubicin (0.75 μM). Cells were isolated 6 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 greater than corresponding value in lapatinib alone cells; **P < 0.05 greater than corresponding non-DOX treated cells; %P < 0.05 less than corresponding value in WT cells. (E) BT474 cells (WT, wild type; AR, anoikis-resistant) in triplicate were treated for 24 h with vehicle or with sodium valproate (750 μM). Cells were washed and the media was replaced with drug free media. Cells were then treated with vehicle (VEH, DMSO) or with [lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM)] and with as indicated doxorubicin (0.75 μM) or irradiated (n.b. 2 Gy). Cells were isolated 24 h later and viability determined by trypan blue (± SEM, n = 3) *P < 0.05 difference between value and LAP+GX greater than corresponding value in vehicle only-treated AR-BT474 cells.](/cms/asset/3da1f9c2-184a-46e4-8b49-2211f5bd31ab/kcbt_a_10926234_f0007.gif)
Figure 7. HDAC inhibitor treatment restores the autophagy response of AR-BT474 cells. (A) BT474 (WT, wild type,; AR, anoikis-resistant) cells were transfected with a plasmid to express LC3-GFP and with scrambled siRNA (siSCR, 20 nM) or an siRNA molecule to knock down Beclin1. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. Six hours after drug exposure cells were examined under a fluorescent microscope and the number of intense GFP punctae per cell determined in at least 50 cells (± SEM, n = 3) #P < 0.05 less than corresponding value in siSCR cells. (B) BT474 (WT, wild type; AR, anoikis-resistant) cells were transfected with scrambled siRNA (siSCR, 20 nM) or an siRNA molecule to knock down Beclin1. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM), or the drug combination. Cells were isolated 12 h later and viability determined by trypan blue (± SEM, n = 3) #P < 0.05 less than corresponding value in siSCR cells. (C) BT474 (WT, wild type; AR, anoikis-resistant) cells were treated with vehicle (VEH, DMSO) or lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 6, 12, 24, and 36 h after drug exposure. Immunoblotting was performed to determine the expression of the indicated proteins (n = 3). (D) BT474 (WT, wild type) cells were transfected with a plasmid to express LC3-GFP and with scrambled siRNA (siSCR, 20 nM) or siRNA molecules to knock down BAK, BAX, NOXA, and PUMA, as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Six hours after drug exposure cells were examined under a fluorescent microscope and the number of intense GFP punctae per cell determined in at least 50 cells (± SEM, n = 3) #P < 0.05 less than corresponding value in siSCR cells. (E) BT474 (AR, anoikis-resistant) cells were transfected with a plasmid to express LC3-GFP and with scrambled siRNA (siSCR, 20 nM) or siRNA molecules to knock down BAK, BAX, NOXA, and PUMA, as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Six hours after drug exposure cells were examined under a fluorescent microscope and the number of intense GFP punctae per cell determined in at least 50–100 cells (± SEM, n = 3) #P < 0.05 less than the corresponding value in siSCR cells. (F) BT474 (AR, anoikis-resistant) cells were transfected with a plasmid to express LC3-GFP and with empty vector plasmid (CMV) or plasmids to express BAX, BAK, NOXA, and PUMA as indicated. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Six hours after drug exposure cells were examined under a fluorescent microscope and the number of intense GFP punctae per cell determined in at least 50 cells (± SEM, n = 3) *P < 0.05 greater than corresponding value in CMV cells. (G) BT474 (WT, wild type; AR, anoikis-resistant) cells were transfected with a plasmid to express LC3-GFP. Thirty-six hours after transfection cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), obatoclax (GX, 50 nM) or the drug combination. Six hours after drug exposure cells were examined under a fluorescent microscope and the number of intense GFP punctae per cell determined in at least 50 cells (± SEM, n = 3) *P < 0.05 greater than value in vehicle cells; **P < 0.05 greater than corresponding value in Lap+GX cells.
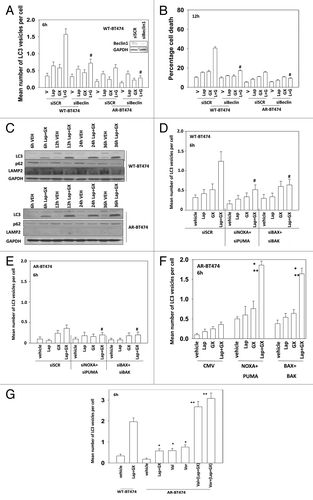
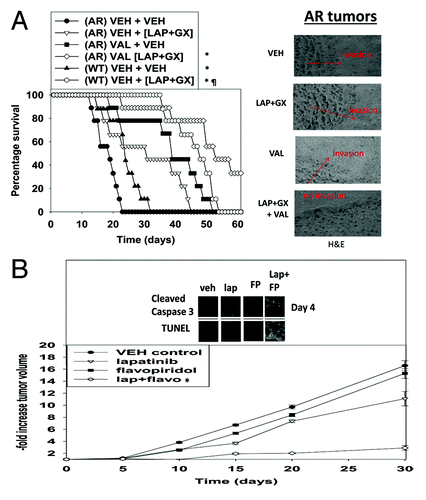
Figure 8. Valproate pre-treatment of AR-BT474 cells growing in mouse brains enhances animal survival. (A) Intracerebral injection of WT-BT474 and AR-BT474 cells was performed over 10 min (1 × 106 cells, each). Seven days after tumor cell implantation, animals were segregated into treatment groups. For animal administration sodium valproate was dissolved in saline. Animals were treated with saline vehicle or sodium valproate to a final concentration of 100 mg/kg QD for 2 d. For animal administration, lapatinib and obatoclax were first dissolved in DMSO, and an equal volume of 50:50 Cremophor EL/ethanol (Sigma-Aldrich) was added. After mixing, a 1:10 dilution was made with sterile PBS. Animals were treated with vehicle (PBS/Cremophor EL/ethanol/DMSO), lapatinib, obatoclax, or a combination of lapatinib and obatoclax using oral gavage to a final concentration of 5 mg/kg QD body mass for obatoclax and 100 mg/kg BID for lapatinib for 3 d. *P < 0.05 greater survival than in AR-BT474 cells with vehicle; ¶P < 0.05 greater survival than vehicle treated WT-BT474 cells. Inset: H&E staining of tumors at the time of animal death. (B) WT-BT474 cells (1 × 106 cells, each animal) were injected into the fourth mammary fat pad. Fourteen days after tumor cell implantation tumors of ~100 mm3 had formed, and animals were segregated into treatment groups. For animal administration, lapatinib and flavopiridol were first dissolved in DMSO, and an equal volume of 50:50 Cremophor EL/ethanol (Sigma-Aldrich) was added. After mixing, a 1:10 dilution was made with sterile PBS. Animals were treated with vehicle (PBS/Cremophor EL/ethanol/DMSO), lapatinib, flavopiridol, or a combination of the drugs using oral gavage to a final concentration of 25 mg/kg QD body mass for flavopiridol and 50 mg/kg BID for lapatinib for 3 d. *P < 0.05 greater survival than in vehicle-treated cells.