Figures & data
Figure 1. HSP70 inhibitors can inhibit autophagy and are cytotoxic to cancer cells. (A) Cell viability analysis (MTT) of A375 melanoma cell line treated with DMSO or the indicated doses of PES, PES-Cl, MKT-077, or VER-155008 for 48 h. The data depicted are representative of 3 independent experiments. Error bars indicate standard deviations. (B) Western blot analysis of apoptosis (cleaved lamin A, cleaved caspase 3) and autophagy (p62/SQSTM1, and LC3 I and II) markers in H1299 and A375 cell lines. Cells were treated with DMSO, 10 uM PES-Cl or MKT-077, or 20 uM VER-155008 for 24 h. Actin is indicated as a loading control. The presence of p62 monomers and oligomers is indicated. (C) Annexin V flow cytometric analysis was conducted on A375 cells treated with the indicated concentrations of PES-Cl, MKT-077, or VER-155008 for 24 h. The data depicted are the average results from 3 independent experiments. Error bars indicate standard deviations. (D) H1299 and A375 cells were treated with DMSO or 10 uM PES-Cl, 10 uM MKT-077, or 20 uM VER-155008 for 24 h. Whole cell lysates were then subjected to immunoblot analysis of HSP90 client proteins using the antibodies indicated. Actin is included as a loading control.
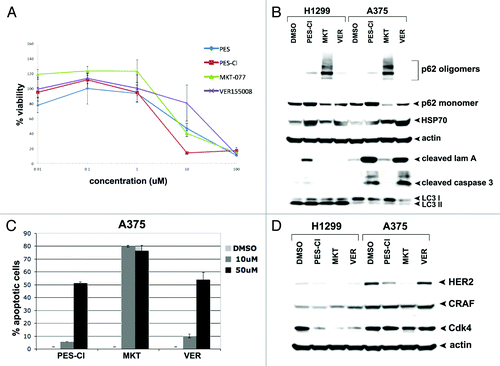
Figure 2. The HSP70 inhibitor PES-Cl inhibits APC/C function and causes G2/M arrest. (A) Western blot analysis of cyclin B1 level in cell-free extracts generated from synchronized HeLa cells treated with DMSO, 10 uM PES-CL, 10 uM MKT-077, or 20 uM VER-155008. The degradation of cyclin B1 by APC/C activity was analyzed at the time points indicated. The data depicted are representative of three independent experiments. (B) Cell cycle analysis of H1299 and A375 cells treated with DMSO, 10 uM PES-CL, 10 uM MKT-077, or 20 uM VER-155008 for 24 h followed by propidium iodide staining and flow cytometry. The graph shows the percentage of cells arrested in G2/M phase of the cell cycle after each treatment. Data are the average results from three independent experiments. Error bars mark standard error.
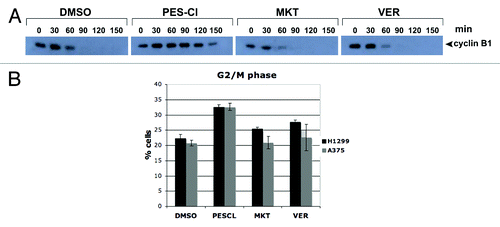
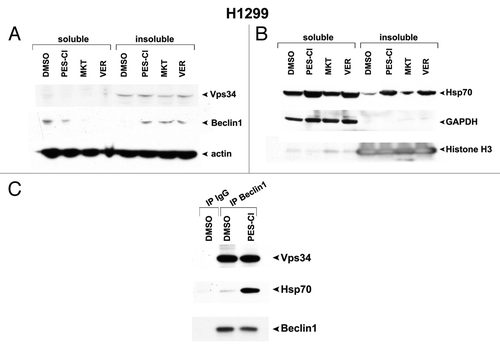
Figure 3. HSP70 inhibitors cause sequestration of Beclin-1 in an insoluble fraction in the cell. (A) Western analysis of Vps34, Beclin-1, and actin in H1299 cells treated with 10 uM PES-Cl or MKT-077 (MKT), or 20 uM VER-155008 (VER) for 24 h followed by fractionation of soluble and insoluble material and analysis by western blot using antibodies for the proteins indicated. Note that Beclin-1 is normally present in a soluble fraction in cells, but that after HSP70 inhibition, this protein changes to an insoluble fraction, indicative of sequestration and inactivation. (B) Western analysis of HSP70, GAPDH (control for soluble protein), and Histone H3 (control for insoluble protein) in H1299 cells treated with 10 uM PES-Cl or MKT-077 (MKT), or 20 uM VER-155008 (VER) for 24 h followed by fractionation of soluble and insoluble material and analysis by western blot using antibodies for the proteins indicated. (C) Immunoprecipitation–western blot analysis of the Beclin1/HSP70 complex in H1299 cells treated with DMSO or 10 uM PES-Cl for 24 h. Five hundred micrograms of lysate was immunoprecipitated with 0.5 ug of antisera to Beclin1, followed by SDS-PAGE, transfer, and western analysis using antisera to Vps34 and HSP70.