Figures & data
Figure 1. The influence of heparin upon the cytotoxicity of chemotherapeutic drugs tested on breast cancer CSCs. CSCs were treated with doxorubicin or tamoxifen or epirubicin respectively at a series of concentrations (as indicated) in the presence or absence of heparin at 1, 5, or 10 U/ml and incubated for 24 h. The cytoxicity was determined using the CyQUANT NF cell proliferation assay kit, based upon the identification of viable cells using a fluorescent dye. In the case of doxorubicin, the MTT assay was used. Asterisks indicate the degree of statistical significance (*P < 0.05; **P < 0.01) as determined in comparing the cytotoxic index of cultures treated with drug alone and to drug in combination with heparin.
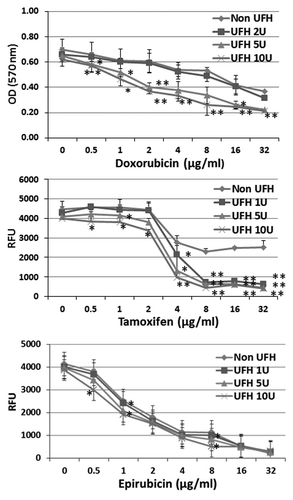
Table 1. Heparin (UFH or LMWH) is able to inhibit the ABC drug transporter system and increase the intracellular accumulation of ABC transporter substrates in cancer cells
Figure 2. The expression of ABC transporter proteins by cancer cell lines. Equivalent amounts of protein in lysates prepared from breast CSCs, lung cancer cell line A549, and breast cancer cell lines MCF-7 and MDA-MB-231, respectively, were subjected to SDS-PAGE followed by western blot analysis with anti-ABCG2 or anti-ABCB1 or anti-ABCC1, respectively. The number shown alongside each sample is the level of expression values, calculated from densitometric analysis in respect of the level of GAPDH in controls.
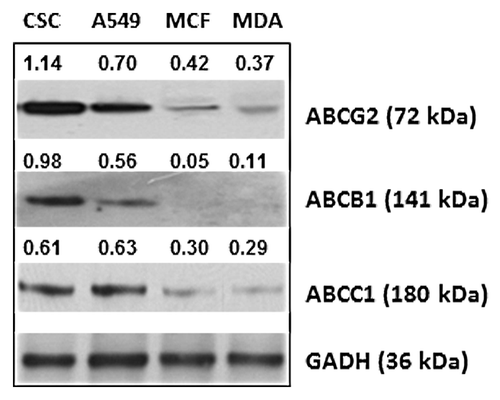
Figure 3. Heparin interaction with ABC transport proteins. (A) The binding of ABC transporter proteins by heparin–agarose. Breast CSCs lysates were incubated with heparin–agarose beads for 2 h, then the beads and supernatant were separated by centrifugation and subjected to western blot analysis using anti-ABCG2, anti-ABCB1, anti-ABCC1, or anti-ABCC2 primary antibody. The results show that heparin has an affinity for ABCG2 (BRCP) and ABCC1 (MRP1), but little or no affinity for ABCB1 (P-gp) and ABCC2 (MPR2). (B) The influence of heparin upon the vanadate sensitive ATPase activity of drug transporter proteins. The ATPase activity of ABCG2, ABCC1, and ABCB1 protein was respectively measured using the specific PREDEASY TM ATPase kit (Solvo Biotechnology, Budapest, Hungary) according to the manufacturer's instructions. ATPase activity was determined in the presence of 1.2 mM sodium orthovanadate either in the absence (termed the activation study) or the presence of substrate (termed the inhibition study) at increasing concentrations of UFH. Significance values (*P < 0.05; **P < 0.01) are shown at a particular heparin concentration where the activity observed in the activation study differed from the activity in the inhibition study (activation/inhibition study are defined by the kit manufacturer). The results show that heparin causes significant inhibition of the ATPase activities of ABCG2, and to a lesser extent that of ABCC1, but has no effect on the activity of ABCB1.
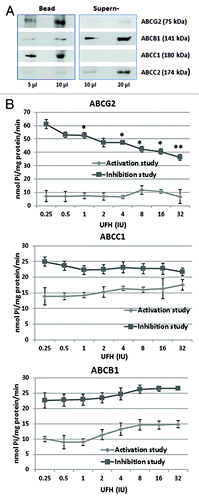
Figure 4. The upregulation of LRP by 17-β-estradiol shows that interaction with LRP was responsible for the heparin induced inhibition of the efflux of doxorubicin from MCF-7 cells. (A) Western blot of non-ABC proteins expression regulated by 17-β-estradiol. MCF-7 cells were treated with 17-β-estradiol with or without heparin for 12 h, lysates were prepared and western blot analysis performed with a range of non ABC protein antibodies, as indicated. The number above each band is the level of expression calculated in respect of the level of GAPDH; (B) Efflux assay demonstrates that the intracellular accumulation of doxorubicin in MCF-7 cell was reduced by 17-β-estradiol treatment. Data shown are the -mean ± SD of 3 separate experiments as calculated relative to the control. *P = 0.01 compared with control cultures. (C). Extraction of cells lysates with heparin–agarose shows that LRP is a heparin binding protein, The experiment was performed as described in . Binding by agarose beads type II are shown as a control.
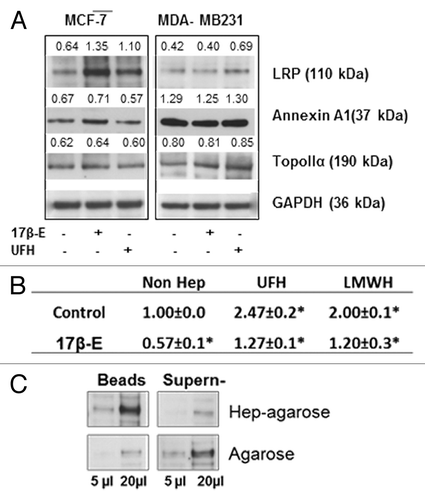
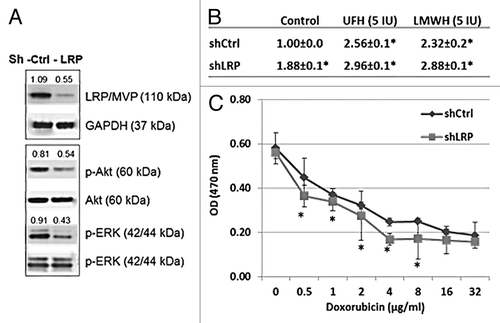
Figure 5. LRP gene knockdown by shRNA demonstrates that LRP is responsible for the transport and cytotoxicity effect of doxorubicin in MCF-7 cells and the doxorubicin transport is inhibited by heparin treatment. (A) Western blot shows the expression of LRP was reduced in the LRP gene knockdown cells following treatment with shLRP. The levels of p-Akt and p-ERK are also reduced in the LRP gene knockdown cells, further evidence of the overall biological-effect of LRP gene knockdown in MCF-7 cells. The number shown are the levels calculated with respect to the level of levels of GAPDH or Akt or ERK in control cells. (B) The intracellular accumulation of doxorubicin was increased in LRP gene knockdown cells. Data represent the fluorescence intensity mean (G mean) ± SD of 3 separate experiments calculated relative to the control. *P = 0.01 compared with control. (C) The toxicity of doxorubicin was reduced in the LRP gene knockdown MCF-7 cells. ED 50 of doxorubicin is 0.75 µg/ml in shControl cells and 1.02 µg/m in shLRP cells.