Figures & data
Figure 1. Measures of clinical toxicity. (A) Percentage of rats with grade 0, 1, 2, and 3 diarrhea between 6 and 120 h following irinotecan (175 mg/kg ip) administration. (B) Percentage change in weight from baseline to 120 h in rats following vehicle control (sorbitol/lactic acid buffer: 45 mg/mL sorbitol/0.9 mg/mL lactic acid, pH 3.4) or irinotecan (175 mg/kg ip). *P < 0.01 vs. 120 h, **P < 0.0001 vs. 24 h and 120 h. A one-way analysis of variance with Tukey’s post hoc was performed to determine significance (n = 39).
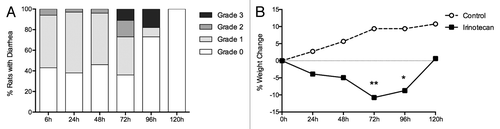
Figure 2. Irinotecan administration causes severe histological damage in the jejunum and colon of DA rats. Characteristic apoptotic bodies are visible at 6 and 96 h post-irinotecan. Gross architectural changes (villous blunting and crypt degeneration) are evident at 24 h, but are most severe at 48 and 72 h hours. Restoration of the epithelium is evident at 120 h, indicated by mitotically active cells. Original magnification 200× (villus) and 400× (crypt).
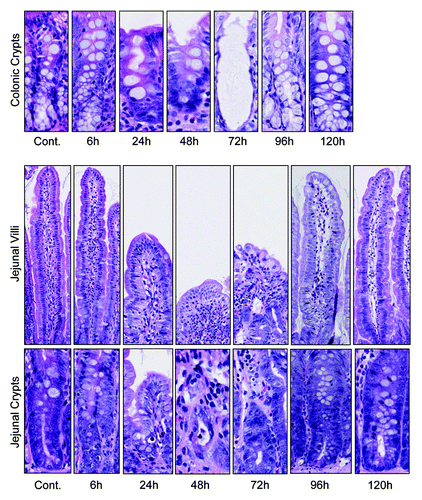
Figure 3. Irinotecan causes molecular defects in all claudin-1 and occludin in the jejunum of the DA rat. There was no statistically significant change in ZO-1 protein expression in the jejunum. Claudin-1 protein expression was significantly decreased 6 h following irinotecan administration, while occludin expression was downregulated in jejunal crypt epithelium 48 h following treatment. ZO-1, claudin-1, and occludin protein expression was analyzed apical villus, basal villus, and crypt epithelium of the jejunum. Staining intensity was analyzed in a blinded fashion (HR Wardill and RJ Gibson) using a validated semi-quantitative grading system.Citation55 A Kruskall–Wallis with a Dunn multiple comparison was performed to determine significance. Data presented as median values (n = 39); ●■□ P < 0.05 vs. control, ●* P < 0.05 vs. 6 h.
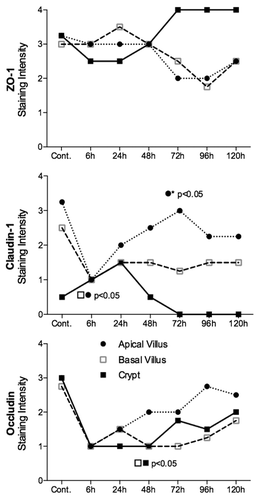
Figure 4. Irinotecan causes tight junction defects in the colon of DA rats. ZO-1 protein expression was significantly decreased in the apical crypt epithelium 96 h following chemotherapy. Irinotecan caused significant downregulation in claudin-1 protein expression in the apical crypt epithelium 24 h following treatment, while a significant decrease was observed 96 h following irinotecan in the crypt crypt epithelium of the colon. Significant downregulation in occludin protein expression was observed at 24 and 48 h following irinotecan administration in the apical and basal crypt epithelium. ZO-1, claudin-1, and occludin protein expression was analyzed in the basal and apical crypt epithelium of the colon. Staining intensity was analyzed in a blinded fashion (HR Wardill and RJ Gibson) using a validated semi-quantitative grading system.Citation55 A Kruskall–Wallis with a Dunn multiple comparison was performed to determine significance. Data presented as median values (n = 39); ■□ P < 0.05 vs. control.
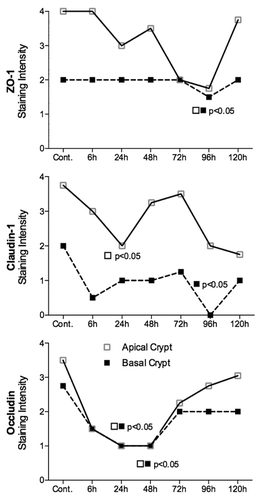
Figure 5. Tight junction protein (ZO-1, claudin-1, and occludin) immunostaining in the colon at selected time points following irinotecan (175 mg/kg ip) administration. Photomicrographs taken at original magnification 400×.
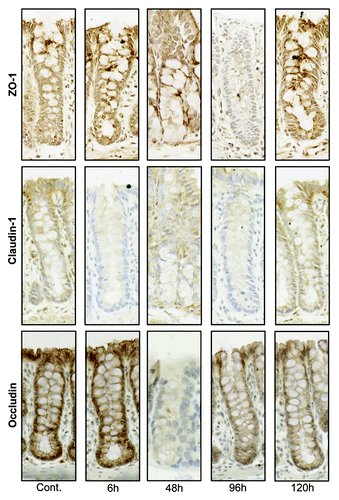
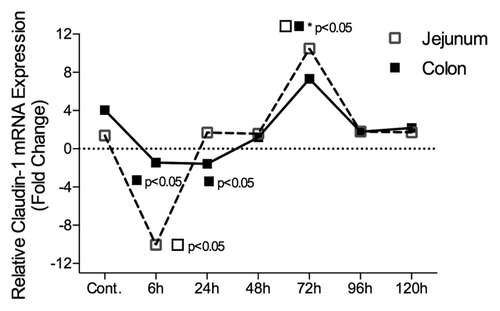
Figure 6. Claudin-1 mRNA expression in the jejunum and colon of irinotecan-treated DA rats. Irinotecan caused an −8.3-fold change in claudin-1 mRNA expression in the jejunum 6 h following irinotecan followed by a 9.8-fold increase at 72 h. A −1.8 and −2.5-fold change in claudin-1 mRNA expression was observed in the colon of irinotecan treated DA rats at 6 h and 24 h, respectively. The fold change in mRNA expression values were used to create the graphs. All data are relative to internal controls (untreated animals, n = 4) and a validated housekeeping gene (UBC). Relative mRNA expression was calculated using the Pfaffl method of relative quantification. A one-way analysis of variance with the Tukey post hoc was performed to determine significance. Data presented as median values (n = 39); ■□ P < 0.05 vs. control, ■□*P < 0.05 vs. 6 h.