Figures & data
Figure 1. pp32 proteins and sphingosine metabolites. (A) 2D chemical structures of various sphingosine metabolites and the synthetic analog FTY720. (B) Molecular weight marker (lanes 1 and 6), pp32 full-length 1–249 (lanes 2 and 7), pp32ΔCT 1–149 (lanes 3 and 8), pp32r1 (lanes 4 and 9), pp32r1Y140H (lanes 5 and 10). Input protein is shown on the left and protein bound to biotin-FTY720 coupled streptavidin beads after washing is shown on the right.
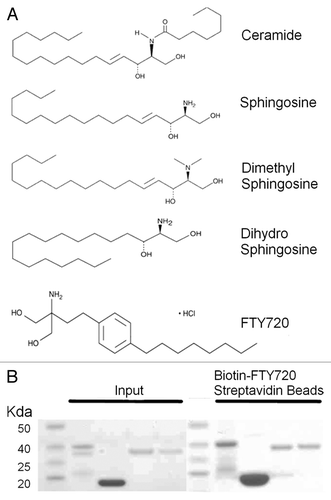
Figure 2. Increased viability of pp32r1 and pp32r1Y140H cell lines treated with FTY720. (A) MTT assay performed on ACHN, ACHN-pp32r1, and ACHN-pp32r1Y140H cell lines treated with 2–16 µM FTY720 and compared with untreated cells. (B) Cells were treated with 6.5 µM FTY720 and visualized by light microscopy. ACHN cells show significant onset of apoptosis while transduced cell lines illustrate only a few cells with apoptotic features. (C) Colony formation assay of untransduced and transduced cell lines treated with 6.5 and 8.5 µM FTY720. (D) A graphical representation of the data shown in (C).
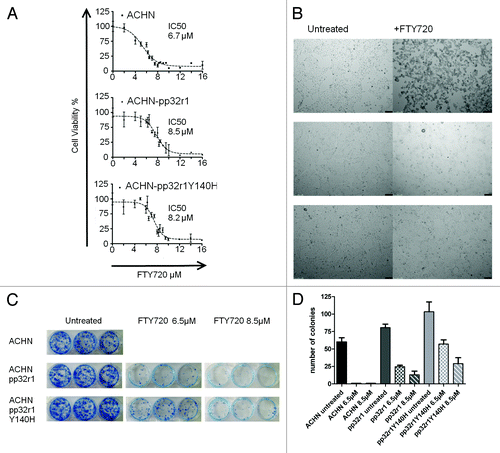
Figure 3. Reduced FTY720 mediated apoptosis in cells overexpressing pp32r1 or pp32r1Y140H. (A) Cells were either untreated, treated with DMSO control, or treated with 6.5 µM FTY720 and stained using an annexinV-FITC/ PI kit. Viable cells (unstained, bottom left quadrant Q4), early apoptotic cells with annexin V-FITC staining (bottom right quadrant Q3), late apoptotic cells with annexin V-FITC/PI staining (top right quadrant Q2), dead cells with PI staining (top left quadrant Q1). (B) The results from (A) for cells treated with 6.5 µM FTY720 are shown graphically as a percentage of viable Q4, apoptotic (early and late Q2/Q3) and dead cells Q1.
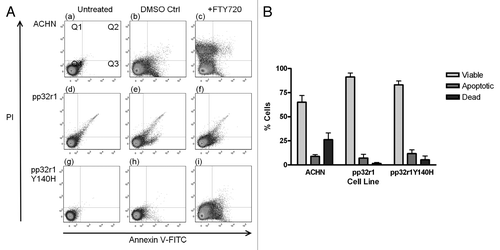
Figure 4. Investigation into the mechanism of pp32r1-mediated FTY720 resistance. (A) Protein phosphatise 2A assay performed on ACHN cell lysates in the absence and presence of FTY720, *P < 0.05. (B) Transduced cell lysates were shown to contain overexpressed pp32r1/pp32r1Y140H (anti-V5) while endogenous pp32 protein is observed in all three lysates. Co-immunoprecipitation,with anti- HuR antibody is only able to co-immunoprecipitate endogenous pp32.
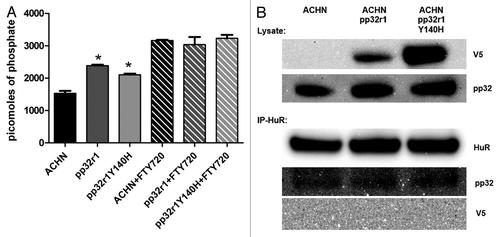
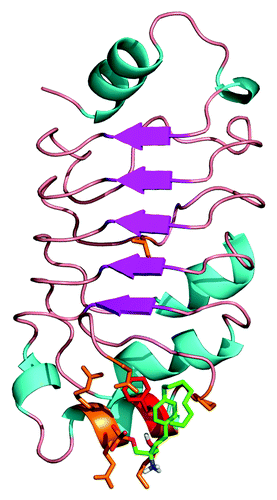
Figure 5. Docking of FTY720 to the crystal structure of the pp32 LRR domain. The structure of the pp322 LRR domain is illustrated as ribbons and colored according to secondary structure. The proposed FTY720 (green) binding site identified by docking studies is illustrated as sticks with residue F136 highlighted in red.