Figures & data
Figure 1. Morphologic characterization of HEV in primary OPSCCs. (A and B) Immunohistochemical staining of CK (AE1/AE3) (blue), MECA-79 (green), and CD31 (red). The MECA-79+/CD31+ blood vessels were detected under the squamous endothelial cells (A) and approximate tumor cells (B). (C) Classic HEVs were located in the lymphocyte-rich areas. (E) high-magnification of the boxed region in (C), classic HEVs kept tall endothelial cells and narrow lumens. (D) HEV-like vessels were adjacent to tumor cells. (F and G) Two consecutive sections of high-magnification of the boxed region in (D). (F) Immunohistochemical staining of MECA-79, HEV-like vessels were dilated and the vessel wall transformed into thin-walled, containing RBC. (G) Immunohistochemical staining of CK (AE1/AE3), which indicated tumor cells. Bar, 100 μm (A and B); 200 μm (C and D), and 50 μm (E–G).
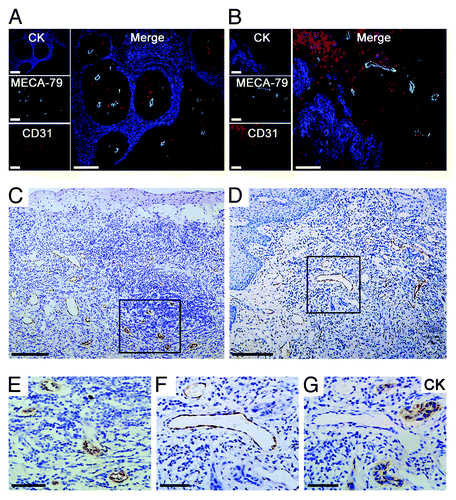
Table 1. Correlation of the density of MECA-79+ vessels and LN metastasis
Figure 2. Quantitative analyses of the remodeling of HEVs and blood vessels in LNs. (A) According to pathologic categories, LNs (n = 308) from OPSCC patients were classified into five groups: nearly-normal LNs (n = 142), reactively-enlarged LNs (n = 63), isolated tumor cells (ITCs) (n = 44), micrometastases (n = 35), and macrometastases (n = 24). Continuous sections from each LN were stained for HEVs (anti-MECA-79) and blood vessels (anti-CD34). Bar, 100 μm. (B) The increase of the mean number of MECA-79+ and CD34+ blood vessels in reactively-enlarged LNs was statistically significant, and the decrease of the mean number in ITCs, micrometastatic and macrometastatic groups was statistically significant. *P < 0.05 vs. normal; **P < 0.001 vs. enlarged. (C) The percentage of MECA-79+/CD34+ blood vessels increased in reactively-enlarged, micrometastatic and macrometastatic LNs, and decreased in ITCs group. *P < 0.001 vs. normal; **P < 0.01 vs. enlarged.
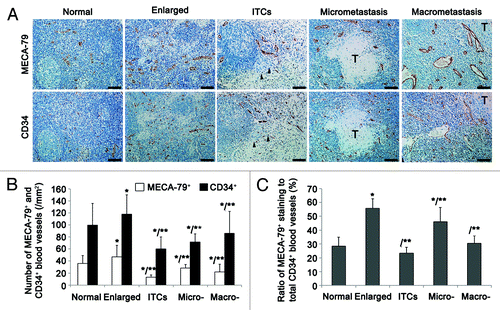
Figure 3. HEVs are implicated in LN metastasis of OPSCC. Metastatic LNs of 30 OPSCC patients were stained for CK (AE1/AE3) (A and C) and MECA-79 antibody (D). (A) Image of a lymphatic-mediated metastatic LN, with a subcapsular tumor embolus (arrow). (C and D) Images of LN consecutive sections, showing tumor embolus in paracortical region (C) where HEVs were present (D). (B) The proportion of lympholymphatic and venolymphatic pathways in 73 metastatic LNs. (E) Images of LN consecutive sections, tumor cells were stained with CK (AE1/AE3) and HEVs stained with HEV-specific marker MECA-79 and pan-endothelial cell marker CD34. Tumor embolus were obverved adjacent to the vascular space lined by plump epithelial cells (arrow). (F and G) Immunohistochemical staining of CK (AE1/AE3) (blue), MECA-79 (green), and CD31 (red). The MECA-79+/CD31+ blood vessels were detected within the tumor lesion (F), and tumor cells migrated to the HEV-dense regions in the front of metastasis (G). Bar, 100 μm (A, F, and G); 200 μm (C and D), and 50 μm (E).
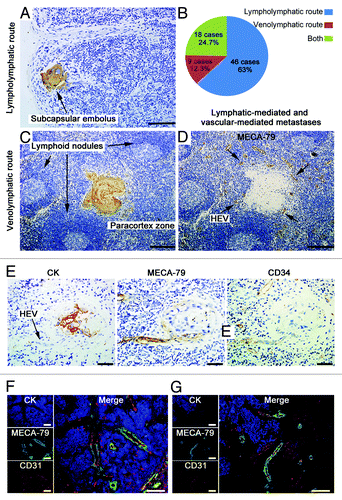
Figure 4. Ultrastructure of HEVs and the surrounding tumor cells in metastatic LNs. (A) Methylene blue-stained epoxy section of a metastatic LN from an OPSCC patient, showing tumor cells (marked by yellow lines) adjacent to a dilated HEV (marked by *). Arrows, the cuboidal epithelial cells of HEVs. (B) Electron microscopy image. Metastatic tumor lesion was adjacent to two HEVs, which displayed thin and enlarged lumens with loose structure. (C) Tumor cells distant to HEVs in a close structure had mass tonofibrils and frequent gap junctions. (D) High-magnification of the yellow boxed region in (B), tumor cells were randomly scattered, and gap junctions decreased or disappeared between adjacent cells. (E) High-magnification of the yellow boxed region in (D), extensive microvilli was observed on the surface of tumor cells, and a few tonofibrils in the cytoplasm. Microvilli of tumor cell adhered to HEV luminal surfaces, and a gap was formed at contact point between tumor cells and endothelial cell membrane (yellow circle). Bar, 50 μm (A), 20 μm (B), 4 μm (C), 10 μm (D), and 2 μm (E).
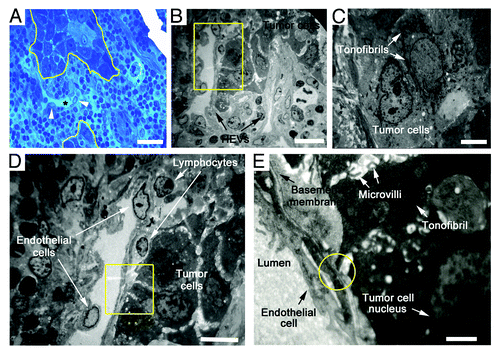
Figure 5. Proliferation rates of the HEV endothelial cells in primary tumor and LNs from OPSCC patients. (A and B) Consecutive sections of a primary tumor, HEVs, and proliferating cells were detected by MECA-79 staining (A) and proliferating marker Ki67 staining (B), respectively. Arrow, the proliferating endothelial cells of HEVs. (C–F) Staining of the proliferating endothelial cells in HEVs (arrows) in reactively-enlarged LN (C), the distant region of metastasis (D), the margin of metastatic tumor lesion (E) and the internal of tumor lesion (F) in metastatic LNs by MECA-79 (green), Ki67 (red), and DAPI (blue). Bar, 50 μm (A–F). (G) The proliferating rates of HEV endothelial cells in primary tumor and LNs. *P < 0.001 vs. control. (H) The proliferating rates of HEV endothelial cells in metastatic LNs. *P < 0.05 vs. control; **P < 0.05 vs. the margin of metastatic tumor lesion.
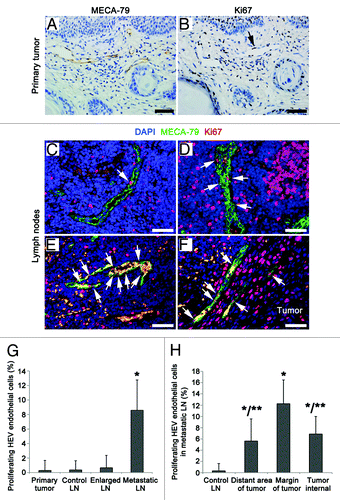
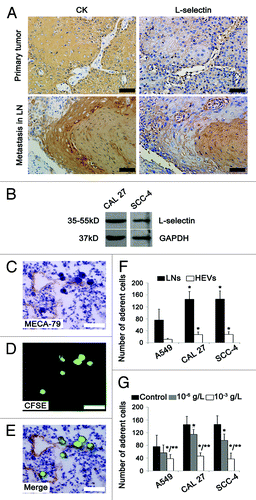
Figure 6. L-selectin is involved in HEV-mediated LN metastasis in OPSCC patients. (A) Immunohistochemical staining of primary and metastatic tumor sections by tumor cells marker CK (AE1/AE3) and adhesion molecule L-selectin. Strong staining of L-selectin was observed in primary and metastatic tumors with membranous and/or cytoplasmic pattern. (B) Western blot analysis of L-selectin expression in human oral cancer cells CAL 27 and SCC-4. GAPDH was loading control. (C–G) Stamper–Woodruff assay of the adherence of human lung adenocarcinoma (A549) and human oral SCC (CAL 27 and SCC-4) cells to LNs and HEVs. (C) HEVs were detected by MECA-79 staining. (D) SCC-4 tumor cells were detected by CFSE (green). (E) Merged images of (C and D), clustered tumor cells adhered to HEVs. Bar, 50 μm (A and C–E). (F) The number of tumor cells adhering to LNs and HEVs. *P < 0.001 vs. A549. (G) L-selectin antibody inhbited the adherence of tumor cells to LNs. Every 200 μl 2 × 106 cell suspension was added: PBS 1 μl (control); 10−6 g/L L-selectin antibody 1 μl; 10−3 g/L L-selectin antibody 1 μl. *P < 0.05 vs. control; **P < 0.05 vs. 10−6 g/L L-selectin antibody.