Figures & data
Figure 1. acALY18 is derived from TRPC1. Western analysis of wild-type (WT) embryonic mouse fibroblasts (A) and TRPC1−/− embryonic mouse fibroblasts (B) treated with 25 nM thapsigargin and probed for acALY18. (C) The representative graph of acALY18 expression in WT (■) and TRPC1−/− (■) embryonic mouse fibroblasts normalized to GAPDH. (D) IL-1β secretion from WT (■) and TRPC1−/− (■) fibroblasts treated with 25 nM thapsigargin for 72 h.
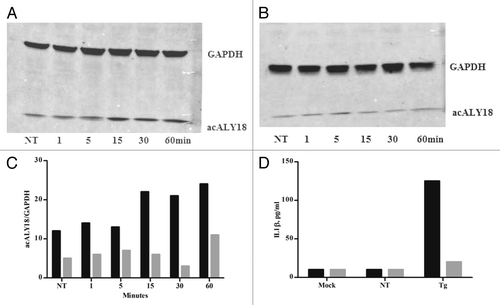
Figure 2. Administration of acALY18 to fibroblasts does not induce ER stress; however, ER stress induces acALY18. (A) A time course measuring the release of acALY18 from TRPC1 when human fibroblasts are treated with 25 nM thapsigargin. Results (top left) show that acALY18 expression precedes GRP78 expression (top right). Western analysis shows that acALY18 does not induce GRP78 in human fibroblasts treated with 25 nM thapsigargin (Tg), acALY18 (5 ng/ml), or mock treatment, for 24 h (bottom). NT, no treatment. (B) Normal human fibroblasts were treated with 25 nM thapsigargin, acALY18 (5 ng/ml), or mock treatment. Western analysis shows phosphorylation of eIF2α (p-eIF2α) after 1 h thapsigargin (Tg) treatment, but not acALY18 or mock treatment (left). Densitometry of the results is plotted (right) as the percentage p-eIF2α of total eIF2α. The expression of IRE1α after 24 h is only detectable in the thapsigargin treated cells (bottom).
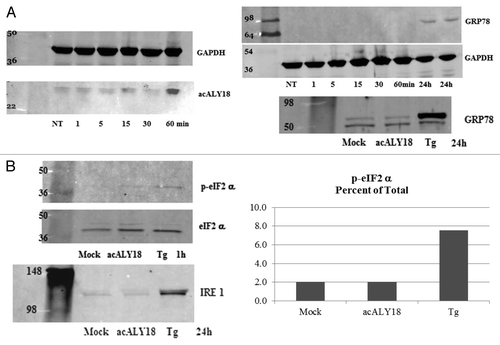
Figure 3. Administration of acALY18 directly to fibroblasts localizes to the cytosol of the cells and appears to form a tetramer. (A) Primary human fibroblasts were treated with 50 nM acALY18-AlexaFluor594 showing intracellular distribution (40× magnification). (B) Exogenously added acALY18 detected by western appears as a tetramer (~8 kDa).
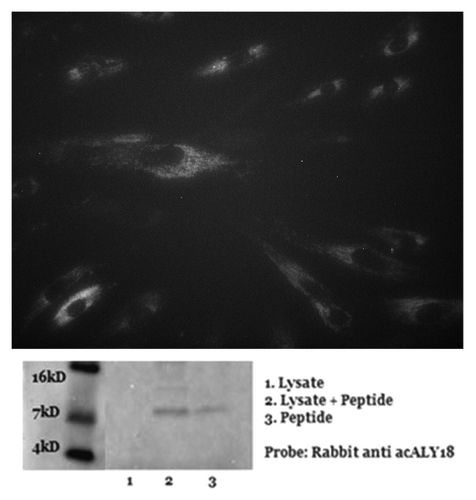
Figure 4. acALY18 forms a disulfide bond with its putative protein target XPB1. (A) Western analysis of human fibroblasts treated with 25 nM thapsigargin and probed for acALY18 (left) or XBP1 (right) at various time points post-treatment. The bar graph of the densitometry measurement shows increased XBP1 (■) expression preceding acALY18 (■) expression. (B) Normal human fibroblasts were treated for 30 min with 25 nM thapsigargin (Tg) and the whole cell lysates were reduced with DTT/BME for 15, 30, or 60 min and analyzed by western. Densitometry measurement of acALY18 levels were normalized to GAPDH (bar graph). NT, no treatment.
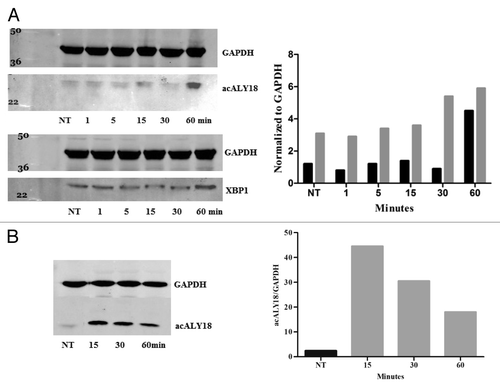
Figure 5. Pulldown assay with XBP1 antibody after 1 and 24 h treatment with 25 nM thapsigargin. (A) Primary human fibroblasts were treated with 25 nM thapsigargin for 1 h or 24 h. Cell lysates were incubated with XBP1 antibody immobilized on Protein-A coated magnetic beads. The bead eluate was analyzed by western and probed for XBP1 (left) and acALY18 (right). Densitometry analysis indicates increasing expression of acALY18 (■) associated with either XBP1u (■) (left) or XBP1s (■) (right) consistent with the whole cell lysate western analysis shown in . (B) Immunoprecipitated protein isolated with immobilized acALY18 antibody and probed with XBP1 antibody (right) or isolated with immobilized XBP1 antibody and probed with acALY18 antibody (left). XBP1 and acALY18 co-localize and acALY18 appears to bind both the spliced and unspliced forms of XBP1.
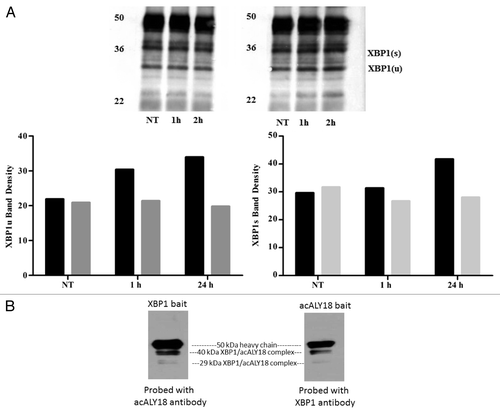
Figure 6. acALY18 abrogates fibrosis. Cells were cultured until confluent. At Day 0 media was removed and saved for hydroxyproline assay. acALY18 (3 ng/ml) was added to fresh media and the cells cultured for 3 or 4 d. At each addition of fresh media, the expired media was saved for hydroxyproline assessment and fresh acALY18 added. The study was terminated at Day 17. (Left) Hydroxyproline levels in all samples were determined at the same time. (Right) Cells were harvested on Day 17, lysed, and protein analyzed by western blotting for α-smooth muscle actin, collagen 3A1, and β-actin.
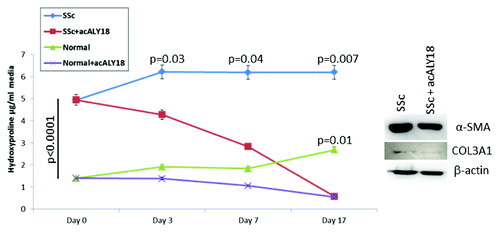
Figure 7. Naclynamide dose response curve. (Top) Dose response curve obtained with primary human fibroblasts cultured for 48 h in the presence of increasing concentrations of Naclynamide. Secreted IL-1β was measured at the 48 h time point by ELISA. Each data point is the mean of 3 separate experiments. Dotted lines represent the regression curve and the calculated EC50 and EC99 from a semi-log plot (middle) and a Hill Plot (bottom) of the data.
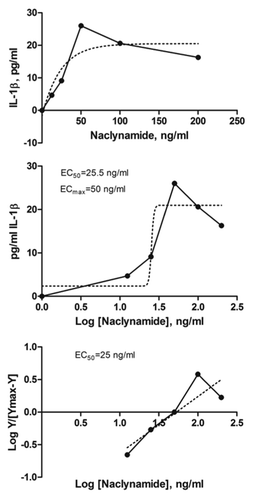
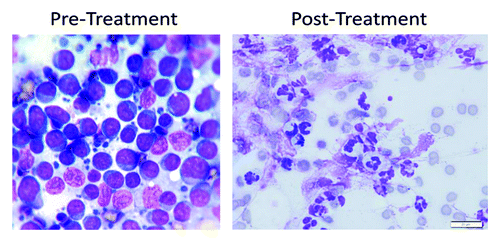
Figure 8. Cytology of the pre-scapular lymph node in the lymphoma patient. Pre-treatment, cytology shows a monomorphic population of lymphoblasts that have round to slightly irregular nuclei in a scant amount of circumferential basophilic cytoplasm. The nuclei also contain large nucleoli. Lymphoglandular bodies are present in the background. Post-treatment cytology shows many degenerate neutrophils, few macrophages, rare reactive fibroblasts, many ruptured cells, abundant streaming nuclear debris, and moderate numbers of erythrocytes. No atypical cells are identified.