Figures & data
Figure 1. Characterization of MS1-TEM1/fLuc and MS1/fLuc endothelial cells. Murine endothelial cells MS1 expressing firefly Luciferase (fLuc) and DsRed (MS1/fLuc) were infected with lentivirus carrying human (h)TEM1 and GFP-Emerald (MS1-hTEM1/fLuc). (A) Quantitative PCR analysis for hTEM1 in H5V and MS1 cells following lentiviral transfection of hTEM1 or control expression cassette. (B) FACS analysis of MS1 cell lines using biotinylated hTEM1-specific antibody MORAb-004 (Ctl: control MS1 cells transduced with empty expression cassette; TEM1: MS1 cells transduced with hTEM1; Ab: MORAb-004). (C and D) MS1-hTEM1/fLuc cells showed a similar luminescent intensity to MS1/fLuc control cells in vitro. Representative image of in vitro bioluminescence study is shown in (C), and summarized in (D). Linearity is observed between plated cell number and bioluminescence. (E and F) MS1/fLuc murine cell injection in vivo formed hemangiomas four weeks following injection of 5 × 102–5 × 105 cells subcutaneously (s.c.) in the flank of nu/nu mice. Representative image of in vivo bioluminescence study is shown in (E) and summarized in (F). In vivo optical imaging can readily detect as few as 5 × 102 cells.
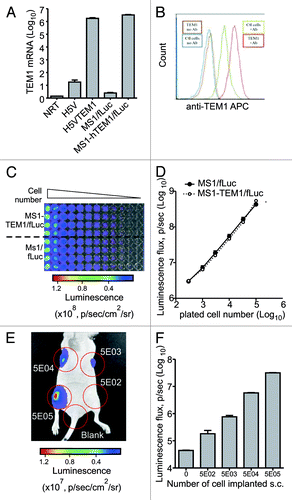
Figure 2. Characterization of the in vivo chimeric vascular-tumor graft model. Chimeric grafts were produced in nu/nu mice by s.c. injection of 106 ID8 cells alone or by implantation of 5 × 106 MS1-TEM1/fLuc or control MS1/fLuc cells mixed with 5 × 105 ID8 tumor cells in 100 μL of serum-free cell culture media. (A) Optical imaging detects chimeric ID8 tumors enriched with MS1/fLuc and MS1-hTEM1/fLuc endothelial cells, while as expected, ID8 tumors lacking MS1/fLuc cells could not be visualized. (B) Representative images of gross vascularization of ID8 tumors alone or those tumors enriched with MS1/fLuc or MS1-hTEM/fLuc endothelial cells. (C) Hemoglobin content of ID8 tumors with or without additional MS1 endothelial cells (n = 7). *P < 0.05; ns, no significance by Student t test. (D) Immunostaining (IHC) with biotinylated TEM1 mAb MORAb-004-biotin shows TEM1-staining of MS1-TEM1/fLuc cells forming capillaries (arrowhead, D).
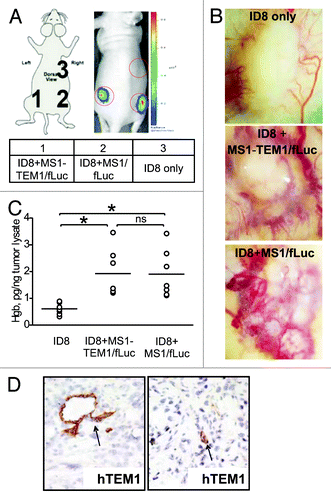
Figure 3. TEM1-specific targeting in vivo by MORAb-004. (A) Subcutaneous (s.c.) MS1 angioma grafts were produced in nu/nu mice by a subcutaneous injection of 5 × 106 MS1-hTEM1/fLuc or control MS1/fLuc cells in 100 μL of serum-free cell culture media. MORAb-004 (5 mg/kg) was injected intraperitoneally (i.p.) at day 0 and every 48 h thereafter for 2 wk. Optical bioluminescence imaging was performed every other day to monitor the survival of MS1 endothelial cells expressing fLuc (n = 8). Representative images from one animal with both MS1-hTEM1/fLuc (arrow) and control MS1/fLuc cells (red arrowhead) at indicated time points are shown in (B); (C) Admixed ID8 + MS1 s.c. grafts were produced in nu/nu mice. MORAb-004 (5 mg/kg) was injected i.p. at day 0 and every 48 h, and optical bioluminescence imaging were performed every other day to monitor the survival of MS1/fLuc endothelial cells (n = 8). After 2 wk, animals bearing chimeric ID8 + MS1 tumor grafts were euthanized and a photograph of the graft was taken ex vivo (D).
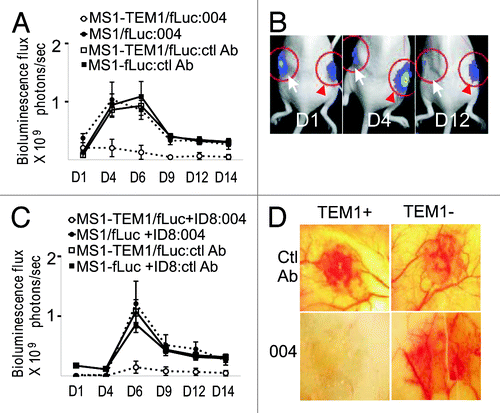
Figure 4. In vitro binding of [125/124I]-MORAb-004 to live cells expressing human TEM1. The binding of radioiodinated MORAb-004 mAb ([125I]-MORAb-004) to hTEM1 was assessed in live cells expressing hTEM1 (MS1-hTEM1/fLuc) compared with control cells not expressing hTEM1 (MS1/fLuc). (A) Cell surface binding of radioiodinated hTEM1 mAb [125I]-MORAb-004 was determined by live-cell radioimmunoassay (RIA). Adherent cells were incubated with increasing concentrations of [125I]-MORAb-004 for 2 h at 4 °C, and binding data were plotted as [125I]-mAb molecules bound per cell ([125I]-mAb/cell) (with Scatchard binding plot as inset). The binding affinity (Kd) of [125I]-MORAb-004 and corresponding Bmax per cell were calculated based on data points fitting as described in Materials and Methods. (B) The competitive inhibition of TEM1 binding was determined by co-incubation of [125I]-MORAb-004 with increasing concentrations of unlabeled MORAb-004 with cells for 2 h at 4 °C. Binding data were plotted as [125I]-mAb molecules bound per cell (mAb/cell), and the IC50 for MORAb-004 was determined by fitting this data to a four-parameter fit as described in Methods. (C) Immunoreactivity (IR) of [124I]-MORAb-004, was determined by Lindmo assay, with serial dilutions of cell suspension incubated with 10 ng of [124I]-MORAb-004 for 1 h at 37 °C. IR, by this method, was >97%. The radiolabeling efficiency for [124I]-MORAb-004 and [125I]-MORAb-004 was calculated as is totally activity bound/total activity in reaction. Radiochemical purity was determined by a trichloroacetic acid precipitation assay. Using a Nanodrop spectrophotometer (Thermofisher) to determine mAb concentrations post-labeling, the [125/124I]-mAb specific activities were reporter as mCi/mg.
![Figure 4. In vitro binding of [125/124I]-MORAb-004 to live cells expressing human TEM1. The binding of radioiodinated MORAb-004 mAb ([125I]-MORAb-004) to hTEM1 was assessed in live cells expressing hTEM1 (MS1-hTEM1/fLuc) compared with control cells not expressing hTEM1 (MS1/fLuc). (A) Cell surface binding of radioiodinated hTEM1 mAb [125I]-MORAb-004 was determined by live-cell radioimmunoassay (RIA). Adherent cells were incubated with increasing concentrations of [125I]-MORAb-004 for 2 h at 4 °C, and binding data were plotted as [125I]-mAb molecules bound per cell ([125I]-mAb/cell) (with Scatchard binding plot as inset). The binding affinity (Kd) of [125I]-MORAb-004 and corresponding Bmax per cell were calculated based on data points fitting as described in Materials and Methods. (B) The competitive inhibition of TEM1 binding was determined by co-incubation of [125I]-MORAb-004 with increasing concentrations of unlabeled MORAb-004 with cells for 2 h at 4 °C. Binding data were plotted as [125I]-mAb molecules bound per cell (mAb/cell), and the IC50 for MORAb-004 was determined by fitting this data to a four-parameter fit as described in Methods. (C) Immunoreactivity (IR) of [124I]-MORAb-004, was determined by Lindmo assay, with serial dilutions of cell suspension incubated with 10 ng of [124I]-MORAb-004 for 1 h at 37 °C. IR, by this method, was >97%. The radiolabeling efficiency for [124I]-MORAb-004 and [125I]-MORAb-004 was calculated as is totally activity bound/total activity in reaction. Radiochemical purity was determined by a trichloroacetic acid precipitation assay. Using a Nanodrop spectrophotometer (Thermofisher) to determine mAb concentrations post-labeling, the [125/124I]-mAb specific activities were reporter as mCi/mg.](/cms/asset/6a8a9b63-f762-4e24-8ac0-bae5ff2dc56c/kcbt_a_10927825_f0004.gif)
Table 1. Biodistribution of radioiodinated [125I]-MORAb-004 in ID8+MS1-hTEM1/fLuc and ID8+MS1/fLuc tumor bearing mice at 1, 4, and 24 h p.i.
Figure 5. In vivo ImmunoPET imaging of tumor-bearing mice with [124I]-MORAb-004. (A) Nude mice bearing tumors developed with ID8 cells and MS1-hTEM1/fLuc cells (arrow) or control tumors developed with ID8 cells and MS1/fLuc cells (red arrowhead) were subject to bioluminescence imaging two weeks following s.c. cell inoculation. (B and C) Small animal PET imaging of the same mice were conducted (B) 18 h, and (C) 6 d post-injection of [124I]-MORAb-004 (~80 μCi, 5 μg per mouse). Mice were pre-fed KI for 24 h prior to PET. Serial posterior transverse (top row) and coronal (bottom row) PET images are shown (0.5 mm thick slices). A strong 124I-PET signal is present in the hTEM1-postive tumor (arrow), with clear delineation. No uptake could be appreciated in the hTEM1-negative tumor. (D and E) Estimated activities and tissue uptake ratios as measured by PET from ROIs drawn on tumors, liver, and soft tissue at 18 h (D) and 6 d (E) post-injection of [124I]-MORAb-004.
![Figure 5. In vivo ImmunoPET imaging of tumor-bearing mice with [124I]-MORAb-004. (A) Nude mice bearing tumors developed with ID8 cells and MS1-hTEM1/fLuc cells (arrow) or control tumors developed with ID8 cells and MS1/fLuc cells (red arrowhead) were subject to bioluminescence imaging two weeks following s.c. cell inoculation. (B and C) Small animal PET imaging of the same mice were conducted (B) 18 h, and (C) 6 d post-injection of [124I]-MORAb-004 (~80 μCi, 5 μg per mouse). Mice were pre-fed KI for 24 h prior to PET. Serial posterior transverse (top row) and coronal (bottom row) PET images are shown (0.5 mm thick slices). A strong 124I-PET signal is present in the hTEM1-postive tumor (arrow), with clear delineation. No uptake could be appreciated in the hTEM1-negative tumor. (D and E) Estimated activities and tissue uptake ratios as measured by PET from ROIs drawn on tumors, liver, and soft tissue at 18 h (D) and 6 d (E) post-injection of [124I]-MORAb-004.](/cms/asset/8a73b36d-4dac-40f3-a4f3-7c88bb14d070/kcbt_a_10927825_f0005.gif)
