Figures & data
Figure 1. Growth inhibition by GSK-3 inhibitor AR-A014418 in neuroblastoma cells. (A) NGP, (B) SH-5Y-SY cells were incubated with AR-A014418 for up to 6 days at various concentrations and cell viability was measured by both MTT assay and colony formation assay. Significant reduction in both number and size of the colonies were seen in AR-A014418 treatment (C and D).
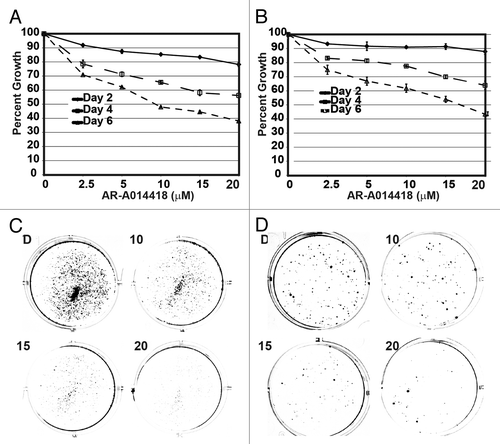
Figure 2. Phosphorylation of GSK-3α reduction is associated with decreased expression of NE markers. Western analysis showing that decreased phosphorylation of GSK-3 α at Tyr 279 compared to GSK-3β phosphorylation at Tyr216 by AR-A014418. In addition, there is a reduction in GSK-3β phosphorylation at ser9. This decrease in phosphorylation reduced the expression of β-catenin, ASCL1, and CgA in NGP (A) and SH-5Y-SY (B) cells. D, DMSO-treated control. GAPDH was used as a loading control.
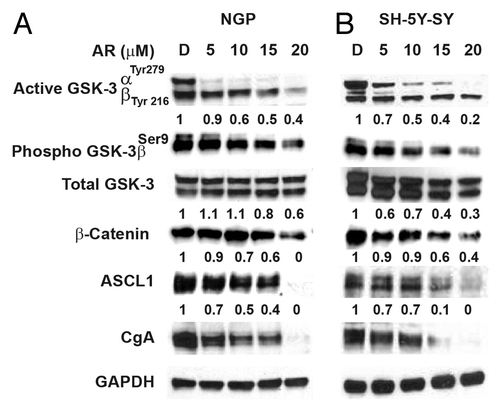
Figure 3. AR-A014418 attenuation of apoptosis inhibitor expression in NGP and SH-5Y-SY cells. Western blot analysis showed there is increase in cleaved PARP, a marker for apoptosis, This was associated with reduction in anti-apoptotic protein Mcl-1 and survivin. D, DMSO-treated control. GAPDH was used as loading control.
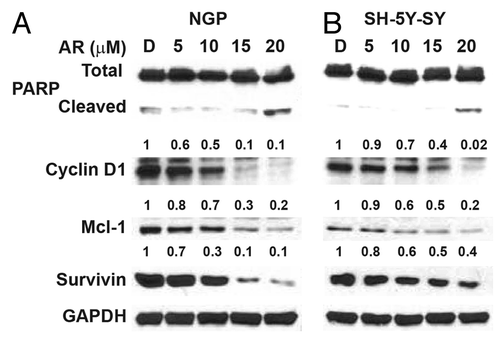
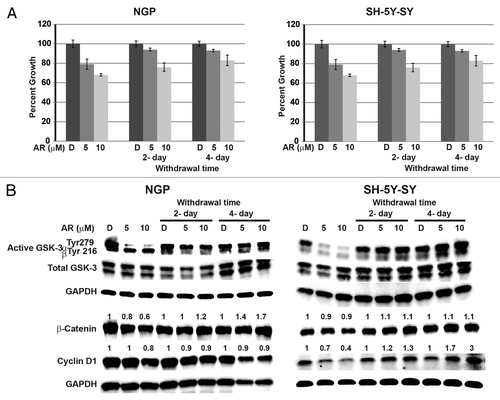
Figure 4. Continuous treatment of AR-A014418 is required for growth suppression of NGP and SH-5Y-SY cells. Cells were treated with AR-A014418 for 4 d and then media was changed to complete media without AR-A014418 for up to 4 d. Cell viability was measured by MTT assay (A) and western blot analysis for the levels of GSK-3 phosphorylation, β-catenin, and cyclin D1 (B). Significant reduction in cellular proliferation was observed with AR-A014418 treatment whereas cellular growth is increased compared to the treatment when the medium was changed to regular medium without AR-A014418 (A) in both cell lines. Importantly, reversal of phosphorylation of GSK-3α protein and increase in β-catenin and cyclin D1 protein was seen in withdrawal condition (B).