Figures & data
Figure 1. Combined effects of LB cultured EcN SNs and 5-FU (µM) on the viability of IEC-6 cells for 24 h (A) or 48 h (B) Cells were treated with DMEM, dried LB re-suspended in DMEM (LB-) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, or dried EcN SN grown in LB re-suspended in DMEM (LB+) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, either alone or in combination with 5-FU (1.25 µM). Data are expressed as percentage of viable cells relative to untreated cell controls. Data are presented as means ± SEM of three independent experiments (n = 9). Bar data not sharing the same letter are significantly different (P < 0.05). *Indicates a significant difference compared with 5-FU control in all 5-FU treatment groups (P < 0.05).
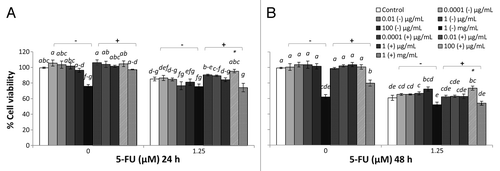
Figure 4. Combined effects of M17 broth supplemented with 10% (v/v) lactose solution (M17) broth cultured EcN SN and 5-FU (µM) on viability of IEC-6 cells for 24 h (A) or 48 h (B). Cells were treated with DMEM, dried M17 broth re-suspended in DMEM (M17-) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, or dried EcN SN had grown in M17 broth re-suspended in DMEM (M17+) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, either alone or in combination with 5-FU (1.25 µM). Data are expressed as percentage of viable cells relative to untreated cell controls. Data are presented as means ± SEM of three independent experiments (n = 9). Bar data not sharing the same letter are significantly different (P < 0.05). *Indicates a significant difference compared with 5-FU control in all 5-FU treatment groups (P < 0.05).
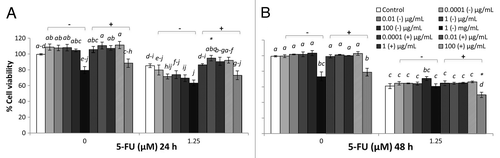
Figure 2. Combined effects of TSB-cultured EcN SN and 5-FU (µM) on the viability of IEC-6 cells for 24 h (A) or 48 h (B). Cells were treated with DMEM, dried TSB re-suspended in DMEM (TSB−) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, or dried EcN SN grown in TSB broth re-suspended in DMEM (TSB+) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, either alone or in combination with 5-FU (1.25 µM). Data are expressed as percentage of viable cells relative to untreated cell controls. Data are presented as means ± SEM of three independent experiments (n = 9). Bar data not sharing the same letter are significantly different (P < 0.05). *Indicates a significant difference compared with 5-FU control in all 5-FU treatment groups (P < 0.05).
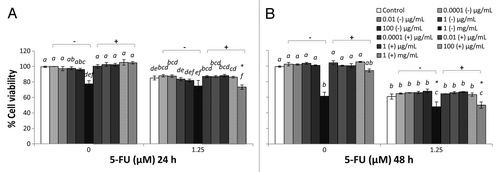
Figure 3. Combined effects of MRS broth cultured EcN SN and 5-FU (µM) on the viability of IEC-6 cells for 24 h (A) or 48 h (B). Cells were treated with DMEM, dried MRS broth re-suspended in DMEM (MRS−) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, or dried EcN SN grown in MRS broth re-suspended in DMEM (MRS+) to reach final concentrations of 0.0001, 0.01, 1, 100 µg/mL, and 1 mg/mL, either alone or in combination with 5-FU (1.25 µM). Data are expressed as percentage of viable cells relative to untreated cell controls. Data are presented as means ± SEM of three independent experiments (n = 9). Bar data not sharing the same letter are significantly different (P < 0.05). *Indicates a significant difference compared with 5-FU control in all 5-FU treatment groups (P < 0.05).
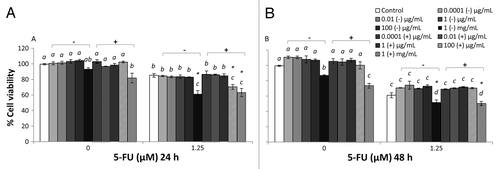
Figure 5. Annexin-PI staining of IEC-6 cells by flow cytometry. DMEM-treated IEC-6 cells are shown in (A) for 24 h and (C) for 48 h. 5-FU treated IEC-6 cells are shown in (B) for 24 h and (D) for 48 h. Results are presented by density plots and separated into four quadrants showing viable cells at lower left, early apoptotic at lower right, late apoptotic/dead at upper right and necrotic at upper left. Values are presented as means of two independent experiments.
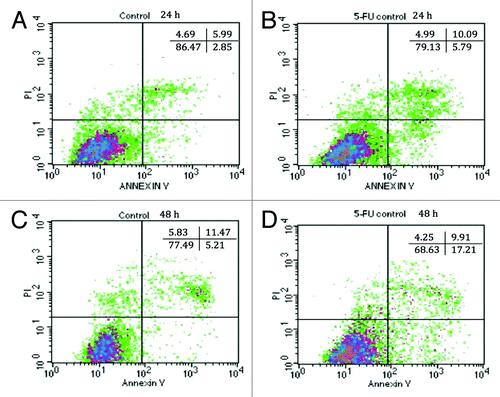
Table 1. Combined effects of EcN SNs and 5-FU (µM) on the percentage of viable (V), apoptotic (A), late apoptotic or dead (LA/D), and necrotic IEC-6 cells at 24 h or 48 h, as measured by flow cytometry
Figure 6. Transepithelial resistance readings (Ω/cm2) of IEC-6 cells over 8 d. IEC-6 cells were cultured with DMEM supplemented with 10% FBS for 8 d. Media was changed every 2–3 d. Measurement was conducted every 2–3 d prior to media change.
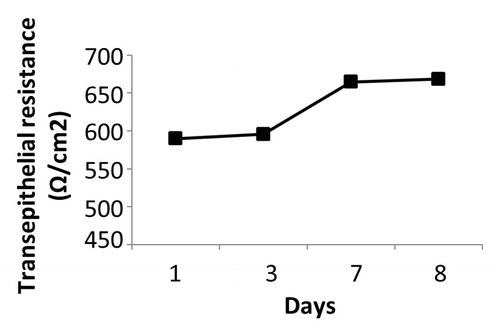
Figure 7. Combined effects of EcN SNs and 5-FU (µM) on the transepithelial resistance (Ω/cm2) of IEC-6 cells for 24 h (A), 48 h (B), 72 h (C), and 96 h (D). Cells were treated with DMEM, or dried EcN SNs (LB+, TSB+, MRS+, or M17+) re-suspended in DMEM to reach final concentration of 100 µg/mL, either alone or in combination with 5-FU (5 µM). TER values are expressed as means ± SEM of three independent experiments (n = 6). Bar data not sharing the same letter are significantly different (P < 0.05). *Indicates a significant difference compared with 5-FU control in all 5-FU treatment groups (P < 0.05).
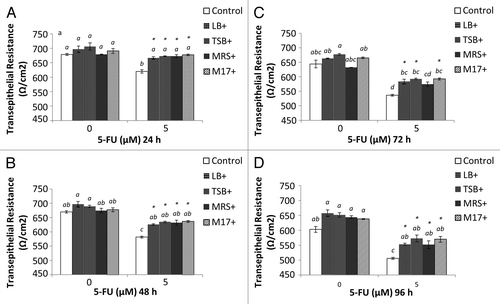
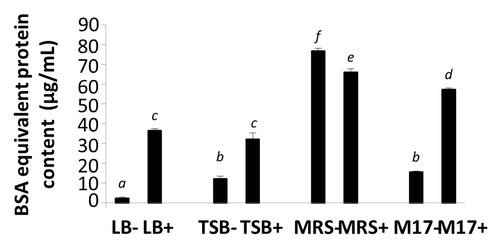
Figure 8. BSA equivalent protein content in media (LB−, TSB−, MRS−, and M17−) and EcN SNs (LB+, TSB+, MRS+, and M17+) were measured by Bradford assay. Data are expressed as means ± SEM of 3 independent experiment (n = 9). Standard error bars not sharing the same letter are significantly different P < 0.05. *Indicates a significant difference compared with other SNs.