Figures & data
Figure 1. Expression of CXCL4 and CXCR3 are homeostatically-regulated in the 5-FU-induced mouse intestinal mucositis. BALB/c mice were treated with 5-FU (250 mg/kg) and sacrificed at 1.5, 3, 5, and 7 d or at 1, 2, 3, 5, and 7 d after the 5-FU treatment. Controls were sacrificed on day 0. The expression of CXCL4 and CXCR3 are shown. (A) The signal intensity of cxcl4 mRNA by gene expression array is presented. (B and C) The expression of cxcl4 and cxcr3 by real-time RT-PCR are presented relative to their baseline at day 0. (D) The CXCL4 and CXCR3 protein expression by western blot are presented as their relative induction over the untreated mice at day 0 after normalization to their respective β-actin loading controls. Data are presented as mean ± SE (n = 3 mice per group). *P < 0.05, **P < 0.01 vs. day 0 controls.
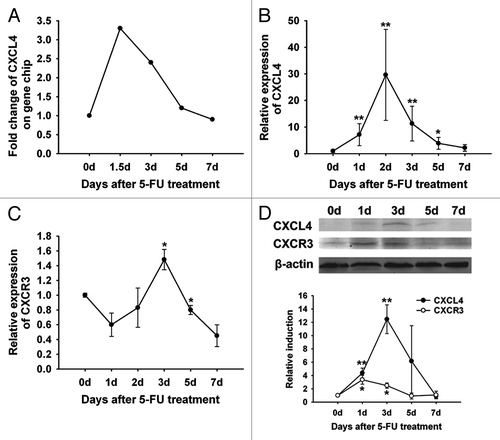
Table 1. Anti-CXCL4 mAb reduces the severity of 5-FU-induced diarrhea in mice
Figure 2. Anti-CXCL4 mAb attenuates the severity of the 5-FU-induced murine intestinal mucositis. (A) Anti-CXCL4 mAb reduced the severity and duration of diarrhea in CIM. Mice were injected with anti-CXCL4 mAb 2 h before 5-FU (250 mg/kg). Diarrhea was scored daily. n = 10 mice per group. (B) Anti-CXCL4 mAb reduced the lethal toxicity of 5-FU. Mice were treated with anti-CXCL4 mAb (1 mg/kg) or equal volume saline 2 h before 5-FU (400 mg/kg) administration. Animal survival for 25 d are presented as Kaplan–Meier survival curves and analyzed by a long-rank test. n = 10 mice per group. (C) Histological and apoptotic presentation of the jejunum. H&E (a–c) and TUNEL staining (d–f) are shown. Anti-CXCL4 mAb was injected 2 h before 5-FU. Mice were sacrificed on day 1 for apoptosis and day 3 for morphometry after 5-FU injection (300 mg/kg). Untreated, normal mice; 5-FU, mice treated with 5-FU only; and 5-FU and anti-CXCL4 mAb, mice treated with 5-FU and anti-CXCL4 mAb, respectively. Average villus length and crypt depth of the mice are shown (g). Twenty villi and crypts were counted per mouse. n = 3 mice per group. A quantitative presentation of the TUNEL-positive cells is shown (h). The average number of TUNEL-positive cells in ten fields per mouse was determined under microscope (400×). n = 3 mice per group. Data are presented as mean ± SD *P < 0.05, **P < 0.01 vs. 5-FU-treated mice.
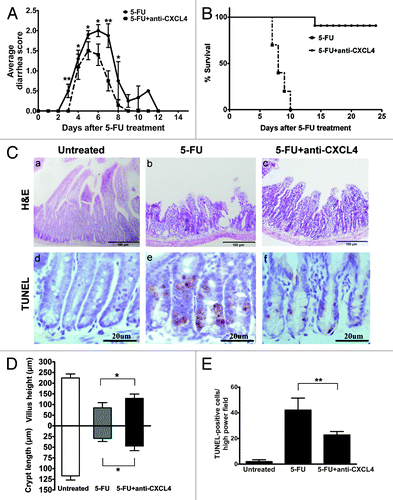
Figure 3. Anti-CXCL4 mAb suppresses the induction of p53 and Bax through CXCR3 to reduce the apoptosis of intestinal epithelia by 5-FU. (A) Representative photomicrographs of apoptotic cells in the jejunum from WT, cxcr3−/−, and p53−/− mice. The mice were treated with 1 mg/kg anti-CXCL4 mAb 2 h before 5-FU (300 mg/kg) and sacrificed at 24 h post-5FU for the TUNEL staining of the apoptotic cells. (d–f) and (A) are identical pictures from the same experiment. (B) Quantitative presentation of the TUNEL-positive cells per crypt is shown in (A). Ten crypts per microscope field and 10 fields per mouse were counted for TUNEL-positive cells. (C) Photographs of the western blot analysis of the jejunum tissues from the mice described in (A) with antibodies against p53 and Bax. (D and E) Quantitative presentation of p53 and Bax is shown in (C). The p53 and Bax levels in the antibody treatment group (5-FU+anti-CXCL4 mAb) are expressed as a percent of their respective controls (5-FU). Data are presented as mean ± SD, n = 3 mice per group. *P < 0.05, **P < 0.01 vs. control group.
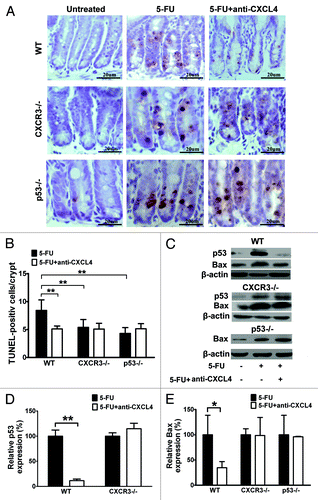
Figure 4. CXCL4 induces apoptosis of an intestinal epithelial cell line IEC-6. (A) The cell proliferation of IEC-6 as a percent of input cells in the cell culture. The IEC-6 cell line cultured with the indicated amount of rhCXCL4 was measured in the MTT assay. (B) Representative photomicrographs of apoptotic IEC-6 cells by TUNEL staining. IEC-6 cells were treated with 0, 5, 10, and 20 μg/mL rhCXCL4 for 24 h. (C) The apoptotic index is defined as the average number of TUNEL-positive cells under 5 continuous microscopic fields (200×). (D) Western blot analysis of p53 and Bax. IEC-6 cells were treated with rhCXCL4 (10 μg/mL) for the indicated times. The relative induction of p53 and Bax over the untreated cells is shown after normalization to their respective β-actin loading controls. (E) Western blot analysis of capases. IEC-6 cells were treated with rhCXCL4 (10 μg/mL) for the indicated time. The anti-capase-8 antibody recognizes the full-length (pro-caspase-8) and the cleaved form of caspase-8. Anti-capase-3 and -9 antibodies can only react with the cleaved forms of caspases-3 and -9. The active forms of the capase-3, -8, and -9 were detected. β-actin was used as the loading control. The data represent mean ± SD from three independent experiments. *P < 0.05, **P < 0.01 vs. control group.
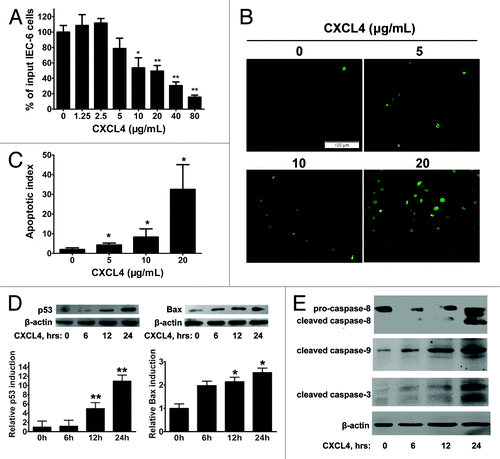
Figure 5. CXCL4-induced expression of p53 and Bax is mediated through activation of p38-MAPK. (A) Western blot analysis of p38-MAPK. IEC-6 cells were treated with rhCXCL4 (10 μg/mL) for the indicated time, and analyzed by western blot using antibodies against phosphor-p38 and p38-MAPK. (B) Western blot analysis of p53 and Bax. The IEC-6 cells were incubated with rhCXCL4 (10 μg/mL) with or without 0.8 μg/mL SB203580, and analyzed by western blot using antibodies against p53, Bax, and β-actin as the loading control. The ratios of p53/β-actin and Bax/β-actin are shown (C). (D) The cell proliferation of IEC-6. IEC-6 cells were treated with the indicated concentrations of rhCXCL4 and SB203580 for 48 h, and analyzed by MTT assay. (C and D) Experiments were repeated three times individually, and the data are presented as mean ± SD *P < 0.05, **P < 0.01 vs. CXCL4-treated group.
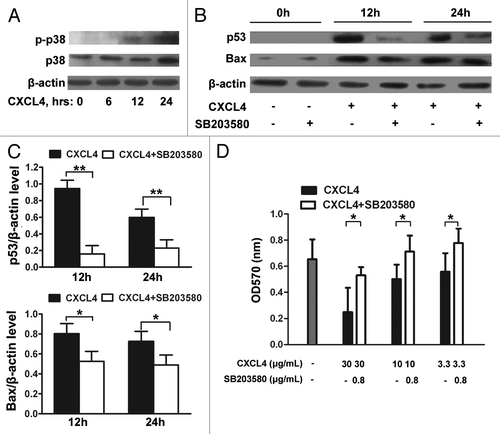
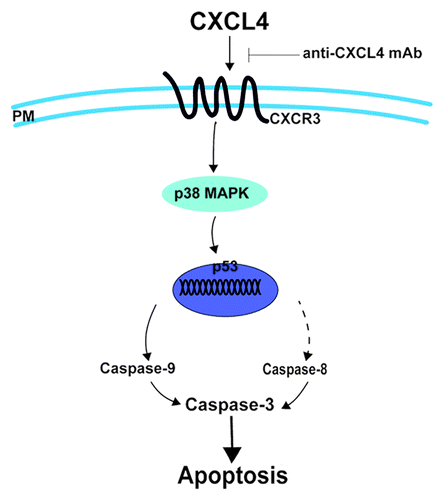
Figure 6. CXCL4 mediates 5-FU-induced apoptosis of the intestinal epithelia. 5-FU induces the local expression of CXCL4, which initiates the intestinal apoptosis pathway. By binding to its CXCR3 receptor, CXCL4 activates p38-MAPK to increase the level of p53, which leads to caspase activation and, ultimately, apoptosis. Anti-CXCL4 mAb effectively blocks the apoptotic role of CXCL4 in 5-FU-induced mucositis, PM: plasma membrane.