Figures & data
Figure 1. Trastuzumab induces phosphorylation of ErbB2-Y1248 and ErbB1-Y845, inhibits Akt phosphorylation and downregulates ErbB3 in trastuzumab-sensitive SKBR3 and BT474 cells. (A) SKBR3 cells (2 × 106) were plated in 10 cm dishes, serum-starved overnight and treated either with trastuzumab (4 μg/mL) or EGF (100 ng/mL) for the indicated times or left untreated. After harvesting, the whole cell lysates (WCL) were incubated with RayBio human EGFR phosphorylation antibody array 1 according to the instructions provided by the manufacturer. The pair of dots in red rectangles indicates phosphorylated ErbB1-Y845 (ErbB1-pY845); blue rectangles indicate ErbB2-pY1248; purple rectangles indicate ErbB2-pY1112. The map of EGFR phosphorylation antibody array 1 is available at http://www.raybiotech.com/. (B) Changes in phosphorylation, as detected by RayBio human EGFR phosphorylation antibody array 1, following treatment of BT474 cells with trastuzumab or EGF for the indicated times. The experimental procedures were essentially the same as described under (A). Red rectangles indicate phosphorylation signal for ErbB1-Y845; blue rectangles indicate phosphorylation signal for ErbB2-pY1248. Black rectangles indicate phosphorylation signal for ErbB2-pT686 and pS1113 (from left to right). The experiments with RayBio human EGFR phosphorylation antibody array 1 were repeated at least twice for both cell lines. (C) SKBR3 cells were serum-starved and incubated either with trastuzumab or EGF for the indicated times. The cells were harvested and the levels of ErbB2-pY1248, ErbB1-pY845, P-Akt-T308, and P-Akt-S473 or P-ERK1/2 and total levels of the indicated proteins in WCL were determined by western blot analysis. Actin western blot analysis of WCL was done to control for equal loading (note: from here on, actin western blots were used to control for equal loading in all figures). Bottom two panels, levels of ErbB1-pY845 and total ErbB1 were determined in ErbB1 immunoprecipitate by western blot analysis. (D) Western blot analysis of BT474 cells treated either with trastuzumab or EGF. The experimental procedures were essentially the same as (C). (E) SKBR3 and BT474 cells were serum-starved overnight and treated with trastuzumab for 1 h or left untreated. The levels of phosphorylated ErbB3-Y1289 (ErbB3-pY1289) and total ErbB3 were detected by western blot analysis using antibodies directed against ErbB3-pY1289 or ErbB3. (F) The cells were grown in the media supplemented with 10% FBS and then treated with trastuzumab (10 μg/mL) for the indicated times. WCL harvested from indicated cells were subjected to western blot analysis. The levels of ErbB3 in WCL were detected using an antibody directed against ErbB3.
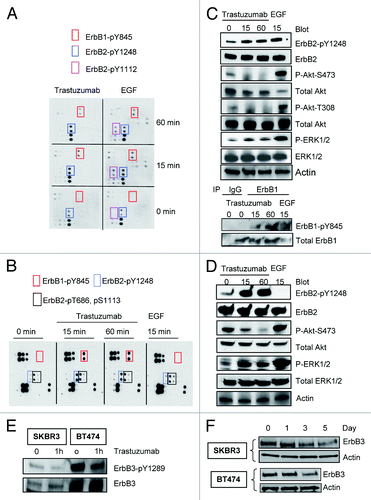
Figure 2. Trastuzumab activates ErbB2 tyrosine kinase and induces ErbB2-Y1248 phosphorylation in the presence of lapatinib. (A) Increased ErbB1-Y845 phosphorylation was detected following trastuzumab treatment of SKBR3 cells. Serum-starved SKBR3 cells were treated for 1 h. After harvesting the WCL, the immunoprecipitation reaction with antibody recognizing ErbB2 (29D8) was performed to detect the heterodimer between ErbB1 and ErbB2. (B) The experiments were performed similar to those described in (A) except that prior to harvesting the WCL, trastuzumab-treated BT474 cells were crosslinked with DTSSP reagent according to the modified protocol provided in the literature.Citation5 (C) Trastuzumab induces the activation of ErbB2 kinase activity in BT474 and SKBR3 cells. After serum-starving overnight, SKBR3 and BT474 cells were either treated with trastuzumab for 1 h or EGF for 15 min, or left untreated as indicated. WCL were harvested and subjected to immunoprecipitation using either human control IgG or trastuzumab. ErbB2 tyrosine kinase activity in each immunoprecipitate was determined using a universal tyrosine kinase assay kit (Takara Bio Inc.) according to manufacturer’s instructions. Data are expressed as mean ± SEM. Statistical significance was determined by the Student t test. *P < 0.05. (D) SKBR3 cells were plated and grown in the serum-containing media and then serum-starved overnight. Cells were either pre-treated with lapatinib (200 nM) for 4 h or not pretreated and then either treated with trastuzumab (4 μg/mL) or left untreated. Analysis of phosphorylation levels of ErbB family phosphorylation sites was done by RayBio human EGFR phosphorylation antibody array 1 according to the manufacturer’s instructions. (E) Analysis of phosphorylation level in ErbB family receptors following treatment of BT474 cells with trastuzumab, lapatinib, or trastuzumab plus lapatinib. The experimental procedures were essentially the same as those described in (D).
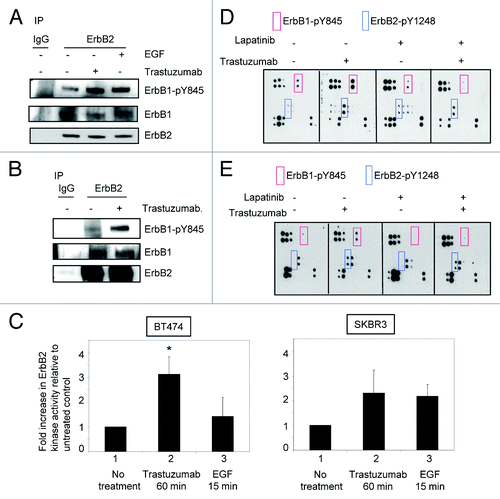
Figure 3. Trastuzumab treatment increases the interaction between ErbB2 and CHK in BT474 cells. (A) BT474 cells were electroporated with either empty pCMV6-entry vector or pCMV-entry vector encoding DDK-tagged CHK. After transfection, cells were grown in the serum-containing media to recover for 24 h, and then were serum-starved overnight. Immunoprecipitation was performed as indicated and the immunoprecipitates were run in parallel with WCL from the indicated reactions to detect CHK and ErbB2 expression by western blot analysis. (B) BT474 cells were plated, serum-starved, fixed, and permeabilized. Following permeabilization, Duolink proximity ligation assay was performed according to the manufacturer’s instructions as indicated in the Materials and Methods. Representative images of samples incubated with anti-ErbB2 and anti-CHK antibodies (top row), and negative control samples (no primary antibodies, middle; anti-ErbB2 antibody only, bottom row).
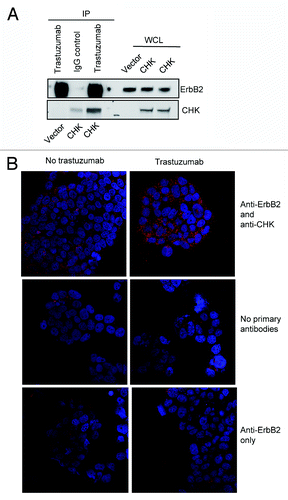
Figure 4. Overexpression of CHK induces ErbB2-Y1248 phosphorylation and mediates ErbB2 degradation following treatment with trastuzumab. (A) BT474 cells were electroporated with either empty pCMV6-entry vector or pCMV-entry vector encoding DDK-tagged CHK. Cells were then grown in serum-containing media to recover for 24 h, followed with serum starvation for 24 h. Vector control cells were either treated with trastuzumab (4 μg/mL) for 1 h or left untreated. The RayBiotech antibody array assay was used to detect the phosphorylation levels among different EGFR family members. Red rectangles, ErbB1-pY845; blue rectangles, ErbB2-pY1248. The expression of DDK-tagged CHK in WCL was detected using an antibody directed against DDK. (B) The experimental procedures were similar to those described in (A) except that WCL obtained from BT474 cells were subjected to western blot analysis to detect ErbB2-pY1248 and total ErbB2. The overexpressed DDK-tagged CHK was immunoprecipitated and detected using antibody directed against DDK. (C) BT474 cell were transiently transfected with either empty pCMV6- entry vector or pCMV6-entry vector encoding DDK-tagged CHK. The cells were grown in the serum-containing media for 24 h and were then serum-starved for 24 h. The cells were either treated with trastuzumab (4 μg/mL) for 1 h or left untreated. The levels of ErbB2-pY1248 and total ErbB2 in WCL were detected using western blot analysis. DDK-tagged CHK expression was detected using an antibody directed against CHK.
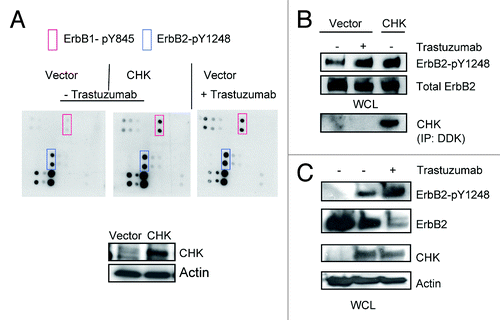
Figure 5. CHK overexpression reduces Akt phosphorylation and inhibits BT474 cell growth. (A) Western blot analysis of Akt phosphorylation in BT474 cells overexpressing either empty pCMV6 vector or DDK-tagged CHK. BT474 cells were electroporated and left to recover in serum containing media for 24 h. Cells were then serum-starved for 24 h and were either treated with trastuzumab (4 μg/mL) or left untreated. WCL were subjected to western blot analysis using antibodies directed against pAkt-S473 or Akt. Anti-DDK antibody was used to detect overexpression of DDK-tagged CHK in WCL. (B) Western blot analysis of Akt-S473 phosphorylation in SKBR3 cells overexpressing either empty pCMV6 vector or DDK-tagged CHK. The experimental procedures were essentially the same as those described in (A). (C) Analysis of growth of BT474 cells transiently overexpressing empty vector or vector encoding DDK-tagged CHK. After transfection, cells were plated in triplicate at 10 × 103/well in 12 well plates in media containing either trastuzumab (10 μg/mL) or no trastuzumab. Cells were trypsinized, mixed with trypan blue and counted using BioRad TC10 automated cell counter on the indicated days. Data represent the mean ± SEM from one of the two independent experiments each performed in triplicate. DDK-tagged CHK expression in BT474 cells was detected by western blot analysis using an anti-DDK antibody.
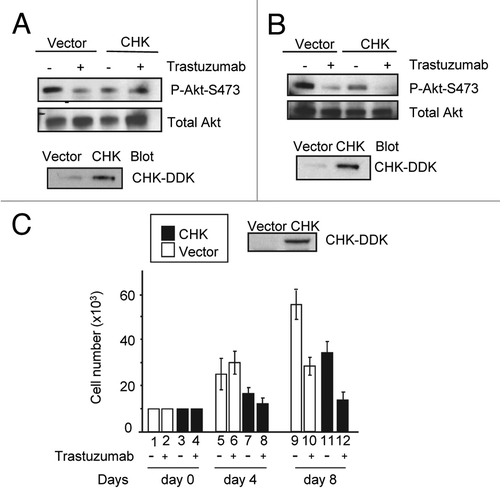
Figure 6. Trastuzumab is unable to induce ErbB2-Y1248 phosphorylation in JIMT1 cells, but is still capable of inhibiting Akt activity. (A) Analysis of ErbB2-Y1248 phosphorylation in trastuzumab-sensitive and trastuzumab-resistant cells as indicated following treatment with trastuzumab. Cells were plated in 10% serum containing media for 24 h and then treated with trastuzumab (4 μg/mL) for 1 h or left untreated. The levels of ErbB2-pY1248, total ErbB2, and endogenous CHK in WCL were detected by western blot analysis using anti-phospho-ErbB2-Y1248, anti-ErbB2, and anti-CHK antibodies. (B) JIMT1 cells were plated at 2.5 × 104/well in 12-well plates in triplicate in media containing either trastuzumab (10 μg/mL) or no trastuzumab (control). Cells were trypsinized, mixed with trypan blue, and counted at the indicated times using a BioRad TC10 automated cell counter. Data represent the mean ± SEM from one of the two independent experiments, and each was performed in triplicate. (C) JIMT1 cells were electroporated with either empty pCMV6-entry vector or pCMV-entry vector encoding DDK-tagged CHK. WCL were harvested 48 h post-transfection and then subjected to immunoprecipitation using an antibody directed against ErbB2. The levels of ErbB2-pY1248 in immuneprecipitates were evaluated by western blot analysis using an antibody directed against ErbB2-pY1248. Total ErbB2 in the immunoprecipitates was detected using an antibody directed against ErbB2. The levels of DDK-CHK in WCL were detected using an antibody directed against DDK tag. IgG was used as a negative control. (D) JIMT1 cells were electroporated with either empty pCMV6-entry vector or pCMV-entry vector encoding DDK-tagged CHK. Cells then were seeded at 10 × 103/well in 12-well plate in triplicate and treated with trastuzumab (50 μg/mL) or left untreated. Cells were trypsinized, mixed with trypan blue and counted using a BioRad TC10 automated cell counter on the indicated days. Data represent the mean ± SEM from one of the two independent experiments, and each was performed in triplicate. Inset: Levels of DDK-CHK expression in WCL were detected in JIMT1cells by western blot analysis using an antibody directed against DDK at the indicated times. (E) JIMT1 cells were plated in media containing 10% serum for 24 h and then serum-starved for another 24 h. Cells then were treated with trastuzumab at the indicated concentration for 1 h or left untreated. The levels of P-Akt-T308 and total Akt in WCL were detected by western blot analysis. (F) The experimental procedures were essentially the same as described in (E) except that SKBR3 and BT474 cells were also included and the levels of P-Akt-S473 and total Akt in WCL were detected by western blot analysis. (G) MCF7 cells were plated at 2.5 × 104/well in a 12-well plate overnight, and then treated with either trastuzumab (4 and 10 μg/mL) or left untreated for the indicated days. Cells were trypsinized, mixed with trypan blue, and counted using BioRad TC10 automated cell counter on the indicated days. Data represent the mean ± SEM from one of the two independent experiments, and each was performed in triplicate. (H) P-ERK1/2 and total ERK1/2 detected in MCF7 WCL by western blot analysis using antibodies directed against P-ERK1/2 and ERK1/2, respectively.
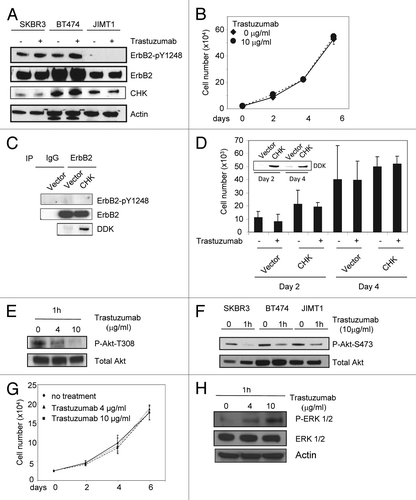
Table 1. Phosphorylation status of ErbB2-Y1248 for trastuzumab non-responders and trastuzumab responders in trastuzumab neoadjuvant settings
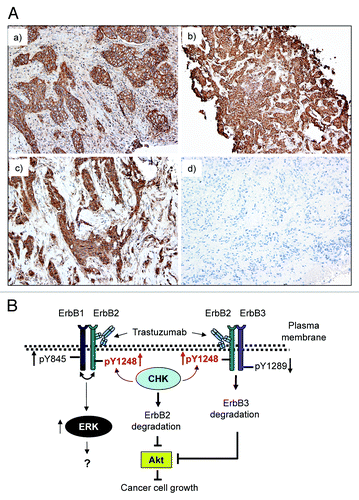
Figure 7. Positive ErbB2-pY1248 staining in ErbB2-positive breast cancer biopsies correlates with the increased trastuzumab response in trastuzumab neoadjuvant settings. (A) Immunohistochemical staining using an anti-phospho-ErbB2-Y1248 antibody on representative cases of core biopsies obtained from breast cancer patients before neoadjuvant trastuzumab treatment. (a and b) Positive with 3+ staining; (c) negative (+, with predominantly cytoplasmic and focal incomplete membrane staining); (d) negative staining. Cases (a and b) had complete pathologic response after trastuzumab treatment. Cases (c and d) had residual disease. (B) Model depicting trastuzumab-mediated interaction between CHK and ErbB2 to regulate ErbB2 phosphorylation at Y1248 and degradation. Overexpression of ErbB2 in trastuzumab-sensitive breast cancer cells leads to heterodimer formation among ErbB family members (ErbB1/ErbB2 and ErbB2/ErbB3). This results in the basal phosphorylation of ErbB2 at Y1248 creating docking sites for downstream effectors such as CHK. When binding to the extracellular domain of ErbB2, trastuzumab stimulates kinase activity of ErbB2, resulting in an increase in ErbB2-pY1248. This in turn promotes recruitment of CHK to ErbB2 via ErbB2-pY1248. Upon binding to ErbB2, CHK further enhances the phosphorylation of ErbB2-Y1248 and induces ErbB2 degradation. Binding of trastuzumab to ErbB2 does not interfere with heterodimer formation between ErbB1 and ErbB2, but rather induces ErbB1-Y845 phosphorylation. This may lead to the induction of ERK1/2 phosphorylation. However, the role of ERK1/2 phosphorylation induced by trastuzumab in trastuzumab-mediated growth inhibition remains elusive. Binding of trastuzumab to ErbB2 in ErbB2/ErbB3 heterodimers leads to the inhibition of ErbB3-Y1289 phosphorylation and promotes ErbB3 degradation. Downregulation of both ErbB2 and ErbB3 results in the inhibition of Akt activity and breast cancer cell growth.