Figures & data
Figure 1. Clodronate treatment reduces tumor burden, TAM levels, and tumor capillary density. (A) Quantification of tumor weight (g) harvested from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes, respectively (n = 4 [SKOV3ip1] and 4 [ID8-VEGF] mice) or equally diluted control liposomes (n = 8 [SKOV3ip1] and 6 [ID8-VEGF] mice) 1 d after last injection showed significantly decreased tumor weight in the clodronate treatment group relative to the control group. **P < 0.01 and ***P < 0.0009 determined by the Student t test. Data are shown as means ± the standard error of the mean (SEM). (B) Quantification of tumor nodules present in the peritoneal cavity of female nude mice in the SKOV3ip1 ovarian tumor model group treated with 0.2 mg/mouse clodronate-encapsulated liposomes (n = 4) or equally diluted control liposomes (n = 8) 1 d after last injection showed significantly fewer number of tumor nodules in the clodronate treatment group relative to the control group. *P < 0.03 determined by the Student t test. Data are shown as means ± SEM. (C) Quantification of F4/80 stained tumor sections taken from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes showed significantly decreased macrophage density in the clodronate treatment group relative to the control group. n = 5 mice for the SKOV3ip1 group and n = 3 mice for the ID8-VEGF group. ****P < 0.0001 and **P = 0.0014 as determined by the Student t test. Data are shown as means ± SEM. (D) Quantification of CD31-positive cells in the tumor sections taken from female mouse SKOV3ip1ovarian tumor models treated with 0.2 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes showed significantly decreased capillary density in the clodronate treatment group relative to the control group (n = 2 mice). ***P = 0.0002 as determined by the Student t test. Data are shown as means ± SEM. (E) Representative histology images of F4/80, and CD31 stained tumor sections taken from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes show decreased macrophage and capillary density in the clodronate treatment group relative to the control group. F4/80-positive cells (staining darkly) represent macrophages within the tissue section. CD31-positive cells (staining darkly) represent endothelial cells within the tissue section. Images were taken at 200× magnification.
![Figure 1. Clodronate treatment reduces tumor burden, TAM levels, and tumor capillary density. (A) Quantification of tumor weight (g) harvested from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes, respectively (n = 4 [SKOV3ip1] and 4 [ID8-VEGF] mice) or equally diluted control liposomes (n = 8 [SKOV3ip1] and 6 [ID8-VEGF] mice) 1 d after last injection showed significantly decreased tumor weight in the clodronate treatment group relative to the control group. **P < 0.01 and ***P < 0.0009 determined by the Student t test. Data are shown as means ± the standard error of the mean (SEM). (B) Quantification of tumor nodules present in the peritoneal cavity of female nude mice in the SKOV3ip1 ovarian tumor model group treated with 0.2 mg/mouse clodronate-encapsulated liposomes (n = 4) or equally diluted control liposomes (n = 8) 1 d after last injection showed significantly fewer number of tumor nodules in the clodronate treatment group relative to the control group. *P < 0.03 determined by the Student t test. Data are shown as means ± SEM. (C) Quantification of F4/80 stained tumor sections taken from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes showed significantly decreased macrophage density in the clodronate treatment group relative to the control group. n = 5 mice for the SKOV3ip1 group and n = 3 mice for the ID8-VEGF group. ****P < 0.0001 and **P = 0.0014 as determined by the Student t test. Data are shown as means ± SEM. (D) Quantification of CD31-positive cells in the tumor sections taken from female mouse SKOV3ip1ovarian tumor models treated with 0.2 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes showed significantly decreased capillary density in the clodronate treatment group relative to the control group (n = 2 mice). ***P = 0.0002 as determined by the Student t test. Data are shown as means ± SEM. (E) Representative histology images of F4/80, and CD31 stained tumor sections taken from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes show decreased macrophage and capillary density in the clodronate treatment group relative to the control group. F4/80-positive cells (staining darkly) represent macrophages within the tissue section. CD31-positive cells (staining darkly) represent endothelial cells within the tissue section. Images were taken at 200× magnification.](/cms/asset/2a97530a-5d12-4f70-93de-01876e0a4504/kcbt_a_10929184_f0001.gif)
Figure 2. Clodronate treatment inhibits cytokine production by endothelial cells and macrophages but has no significant effect on tumor cytokine secretion or cell proliferation. (A) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay shows the response of endothelial cells (RF24) to increasing doses of clodronate at 180 h. Cells showed an IC50 of 3.725 mM (95% CI: 3.540–3.909 mM) determined by interpolation of data. (B) MTT cell viability assay shows the response of response of tumor cells (SKOV3) to increasing doses of clodronate at 72 h. Cells showed an IC50 of 3.941 mM (95% CI: 3.488–4.393 mM) as determined by interpolation of data. (C) Quantification of EGF, FGF2, TGFα, IFNγ, IL6, IL8, IL10, TNFα, and VEGF secretion (pg/mL) by in vitro endothelial (RF24) tumor (SKOV3) cells and activated macrophages (THP-1) treated with media (control) or filtered clodronate salt (2 mM) shows significant decrease in cytokine secretion of endothelial cells in the clodronate treatment group relative to the control group, but shows mixed results in cytokine secretion of activated macrophages and no significant change in cytokine secretion of tumor cells between the clodronate treatment and control groups. Supernatant was collected 48 h after treatment administration for endothelial and tumor cells, and 12 h after treatment administration for activated macrophages. n = 3 samples collected per group, measured in duplicates by ELISA.*P < 0.05, ****P < 0.0001 and NS > 0.05, not significant, as determined by the Student t test. Data are shown as means ± SEM.
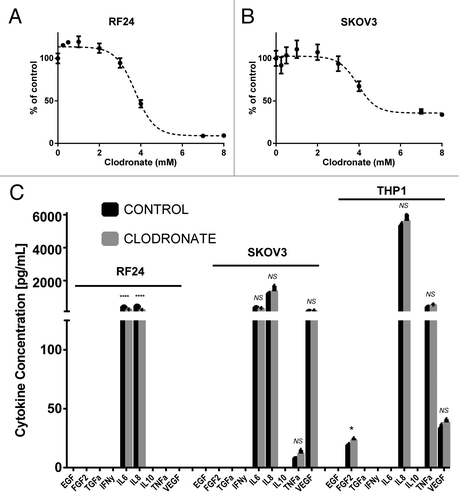
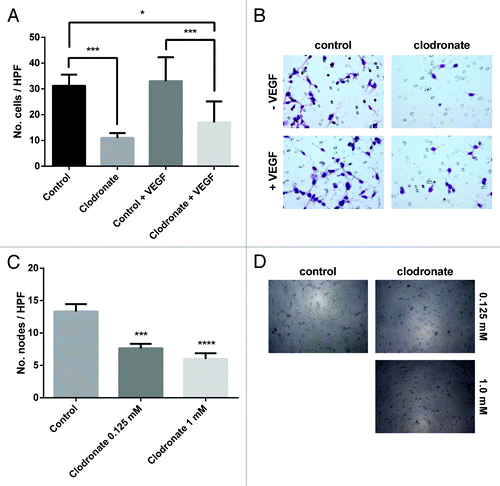
Figure 3. Clodronate impairs capillary recruitment and formation. (A) Quantification of endothelial cell (RF24) migration after treatment with 2 mM clodronate-encapsulated liposomes or equally diluted control liposomes ± VEGF (20 ng/mL) indicates clodronate treatment significantly decreases endothelial cell migration compared with the control group. n = 2 inserts per group, 5 pictures per insert. *P < 0.02, ***P < 0.0007 as determined by the Student t test. Data are shown as means ± SEM. (B) Representative images of migration assay seen in (A) show a decreased number of endothelial cells within the insert of the clodronate treatment group relative to the control group. Images were taken after prior treatment with 2 mM clodronate-encapsulated liposomes, equally diluted liposomes (control), control plus 20 ng/mL VEGF, or 2 mM clodronate-encapsulated liposomes plus 20 ng/mL VEGF. Images were captured at 200× magnification. (C) Endothelial vessel formation was quantified by counting the number of nodes (points at which where three or more elongated cells meet) formed on the gel matrix after prior treatment with 2 mM, 0.125 mM clodronate-encapsulated liposomes or equally diluted control liposomes (to 0.125 mM, control). n = 3 wells per group, 5 pictures per well. ***P < 0.0003, ****P < 0.0001 (compared with media) as determined by the Student t test. Data are shown as means ± SEM. (D) Representative images of 3D tube formation seen in (C) on a gel matrix correlating to capillary formation show decreased vessel formation in the clodronate treatment group relative to the control group.. Images were taken after treatment with 0.125 mM or 2 mM clodronate-encapsulated liposomes or equally dilute liposomes (to 0.125 mM, control). Images were taken at 100× magnification.