Figures & data
Table 1. Toxicity data: Grade ≥3 toxicities and toxicities of special interest
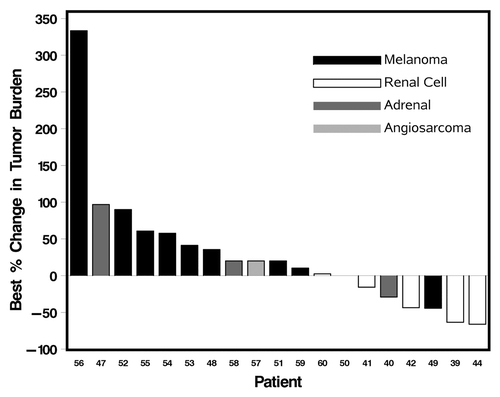
Figure 1. Waterfall plot of best responses. Patients with new lesions were empirically assigned a 20% increase in target tumor measurement for accurate reflection of best response.